The anhydrous magnesium acetate is a white, crystalline, and deliquescent solid; it is the acetate salt form of magnesium. It has the chemical formula Mg(C2H3O2)2 and has the chemical formula Mg(CH3COO)2 • 4H2O in its hydrated form, magnesium acetate tetrahydrate. Magnesium has an oxidation state of 2+ in this compound. White hygroscopic crystals appear as magnesium acetate. It smells like acetic acid and it is water-soluble. Its pH may be considered neutral when it is in an aqueous solution. As a source of magnesium in biological reactions, magnesium acetate is widely used.

(Magnesium Acetate)
The Clamond basket, one of the first powerful gas shells, was invented by Charles Clamond in 1881. Magnesium acetate, magnesium hydroxide, and water were among the reagents used in that invention. For a variety of biochemical processes involved in nerve signaling, bone mineralization, and muscle contractions, magnesium is a divalent cation that is necessary. It must be kept away from water due to the fact that it is extremely hygroscopic. Magnesium acetate is therefore incompatible with, and should not be combined with, strong oxidizers.
In chemical applications, magnesium acetate is widely used as a source of magnesium or as an acetate ion. Magnesium acetate synthesis from magnesium hydroxide reaction with acetic acid:
2 CH3COOH + Mg(OH)2 → (CH3COO)2Mg + 2 H2O
Both molecular dynamics simulations and surface tension measurements were conducted while magnesium acetate and magnesium nitrate were used. In the experiment, the authors observed that compared to the nitrate ion, the acetate had a greater affinity for the surface and that the Mg2+ repelled strongly away from air/liquid interference. They also found that, relative to nitrate, Mg2+ had a greater propensity to bind with the acetate ion.
Along with the release of hydrogen gas, the reaction of metallic magnesium with acetic acid dissolved in dry benzene allows magnesium acetate to form.
Mg +2 CH3COOH → Mg(CH3COO)2 + H2
Magnesium relies on about 350 enzymes involved in glycolysis and the Krebs cycle, the production of cyclic-AMP and ATP, the transduction of cellular signals, and the synthesis of proteins and nucleic acids. A mixture called calcium magnesium acetate (CMA) is one of the most common uses of magnesium acetate. It is a combination of acetate from calcium and acetate from magnesium. In the crystallization of proteins, magnesium acetate has been commonly used. A protocol has been published for the separation of creatine kinase isoenzymes MB and BB and lactate dehydrogenase isoenzyme LD1 that requires elution with magnesium acetate.
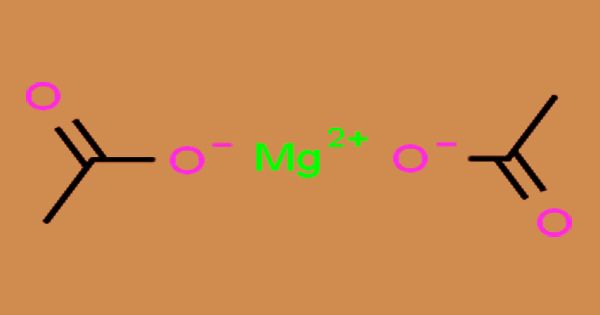
In preparation for fluorine analysis, magnesium acetate was shown to be effective in ashing organic compounds when high or low fluorine concentrations are present. The primary application of magnesium acetate is in the manufacture of rayon fiber, which is used for the towing of cigarette filters. In textile printing, magnesium acetate is also used as a dye fixative, as a deodorant, a disinfectant, an antiseptic in medicine, and as a chemical reagent. It is a reasonably safe compound to handle and a health hazard rating of zero has been given.
However, gloves and safety goggles should still be treated with magnesium acetate. It can cause inflammation in the respective areas if it gets into the eyes, the skin, swallowed, or inhaled: eyes, skin, gastrointestinal system, and lungs. It must be kept away from water due to the fact that it is extremely hygroscopic. It is also incompatible with, and should not be combined with, strong oxidizers.
Information Sources: