Solar fuels are a type of renewable energy that are produced using solar energy to convert water or carbon dioxide into a fuel. There has been significant research in recent years on developing ways to produce solar fuels using a process called artificial photosynthesis, which uses sunlight to convert these substances into fuels such as hydrogen or methanol. One recent development in this field is the use of metal-organic frameworks (MOFs) as a means of capturing and storing solar energy, which can then be used to produce solar fuels. This is a promising step towards the development of renewable energy sources that can be produced using only air and sunlight.
Chemical engineers have created a solar-powered artificial leaf based on a novel transparent and porous electrode capable of harvesting water from the air and converting it into hydrogen fuel. The semiconductor-based technology is both scalable and simple to implement.
For decades, researchers have wished for a device that can harvest water from the air and produce hydrogen fuel while being entirely powered by solar energy. Now, EPFL chemical engineer Kevin Sivula and his team have taken an important step toward making this vision a reality. They have created an ingenious yet simple system that combines semiconductor-based technology with novel electrodes that have two key characteristics: they are porous, allowing for maximum contact with water in the air, and transparent, allowing the semiconductor coating to be exposed to sunlight. When the device is simply exposed to sunlight, it takes water from the air and produces hydrogen gas. The results are published in Advanced Materials.
What’s new? It’s their novel gas diffusion electrodes, which are transparent, porous and conductive, enabling this solar-powered technology for turning water – in its gas state from the air – into hydrogen fuel.
To realize a sustainable society, we need ways to store renewable energy as chemicals that can be used as fuels and feedstocks in industry. Solar energy is the most abundant form of renewable energy, and we are striving to develop economically-competitive ways to produce solar fuels.
Kevin Sivula
“To realize a sustainable society, we need ways to store renewable energy as chemicals that can be used as fuels and feedstocks in industry. Solar energy is the most abundant form of renewable energy, and we are striving to develop economically-competitive ways to produce solar fuels,” says Sivula of EPFL’s Laboratory for Molecular Engineering of Optoelectronic Nanomaterials and principal investigator of the study.
Inspiration from a plant’s leaf
The EPFL engineers, in collaboration with Toyota Motor Europe, were inspired by the way plants convert sunlight into chemical energy by using carbon dioxide from the air in their research for renewable fossil-free fuels. A plant harvests carbon dioxide and water from its surroundings and, with the help of sunlight, can convert these molecules into sugars and starches, a process known as photosynthesis. The energy from the sun is stored in the sugars and starches as chemical bonds.
When coated with a light-harvesting semiconductor material, the transparent gas diffusion electrodes developed by Sivula and his team act like an artificial leaf, harvesting water from the air and sunlight to produce hydrogen gas. The energy of the sun is stored in the form of hydrogen bonds. Instead of using traditional layers that are opaque to sunlight to construct electrodes, they use a 3-dimensional mesh of felted glass fibers.
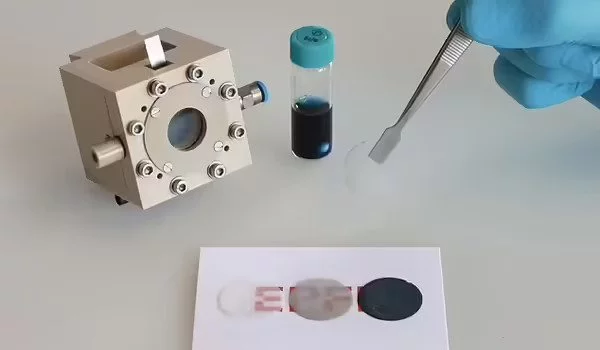
Marina Caretti, lead author of the work, says, “Developing our prototype device was challenging since transparent gas-diffusion electrodes have not been previously demonstrated, and we had to develop new procedures for each step. However, since each step is relatively simple and scalable, I think that our approach will open new horizons for a wide range of applications starting from gas diffusion substrates for solar-driven hydrogen production.”
From liquid water to humidity in the air
Sivula and other research groups have previously demonstrated that artificial photosynthesis is possible by generating hydrogen fuel from liquid water and sunlight using a device known as a photoelectrochemical (PEC) cell. A PEC cell is a device that uses incident light to stimulate a photosensitive material, such as a semiconductor, immersed in a liquid solution, resulting in a chemical reaction. However, for practical purposes, this process has drawbacks, such as the difficulty of producing large-area PEC devices that use liquid.
Sivula wanted to demonstrate that PEC technology could be adapted to harvest humidity from the air instead, which led to the creation of their new gas diffusion electrode. Although electrochemical cells (such as fuel cells) have been demonstrated to work with gases rather than liquids, the gas diffusion electrodes used previously are opaque and incompatible with solar-powered PEC technology.
The researchers are now concentrating their efforts on optimizing the system. What is the optimum fiber size? What is the ideal pore size? What are the best semiconductors and membrane materials? These are the questions being pursued by the EU Project “Sun-to-X,” which aims to advance this technology and develop new methods of converting hydrogen into liquid fuels.
Making transparent, gas-diffusion electrodes
The researchers begin with a type of glass wool that is essentially quartz (also known as silicon oxide) fibers and process it into felt wafers by fusing the fibers together at high temperatures to create transparent gas diffusion electrodes. The wafer is then coated with a transparent thin film of fluorine-doped tin oxide, which is known for its excellent conductivity, robustness, and ease of scaling-up.
These initial steps produce a transparent, porous, and conducting wafer, which is critical for maximizing contact with water molecules in air and allowing photons to pass through. The wafer is then coated once more, this time with a thin film of semiconductor materials that absorb sunlight. This second thin coating still allows light through but appears opaque due to the porous substrate’s large surface area. As is, this coated wafer can already produce hydrogen fuel once exposed to sunlight.
The scientists then built a small chamber to house the coated wafer, as well as a membrane to separate the produced hydrogen gas for measurement. When their chamber is exposed to sunlight under humid conditions, hydrogen gas is produced, proving the scientists’ goal of developing a transparent gas- diffusion electrode for solar-powered hydrogen gas production.
While the scientists did not conduct a formal study of the solar-to-hydrogen conversion efficiency in their demonstration, they acknowledge that it is modest for this prototype and is currently less than that of liquid-based PEC cells. Based on the materials used, the coated wafer’s maximum theoretical solar-to-hydrogen conversion efficiency is 12%, whereas liquid cells have been shown to be up to 19% efficient.