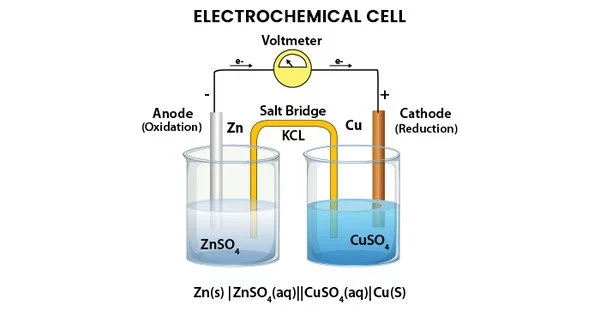
An electrochemical cell is a device that converts chemical energy into electrical energy through a redox reaction. It consists of two electrodes, an anode and a cathode, which are immersed in an electrolyte solution. The anode is the electrode where oxidation occurs, while the cathode is the electrode where reduction occurs.
An electrochemical cell is a device that can produce electrical energy from chemical reactions or use electrical energy to initiate chemical reactions. Electrochemical cells that generate an electric current are known as voltaic or galvanic cells, whereas those that generate chemical reactions, such as electrolysis, are known as electrolytic cells. A standard 1.5 volt cell for consumer use is a common example of a galvanic cell. A battery is made up of one or more cells that are connected in parallel, series, or series-and-parallel fashion.
An electrolytic cell is an electrochemical cell that uses electrical energy to drive a non-spontaneous redox reaction. They are frequently used to break down chemical compounds in a process known as electrolysis—the Greek word lysis means “to break up.”
Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminium and other chemicals. Electroplating (e.g. of copper, silver, nickel or chromium) is done using an electrolytic cell. Electrolysis is a technique that uses a direct electric current (DC).
During the reaction, electrons flow from the anode to the cathode through an external circuit, producing an electrical current. The electrolyte solution, which contains ions that can move freely, completes the circuit by allowing the flow of charged particles between the two electrodes.
There are two types of electrochemical cells: galvanic cells (also known as voltaic cells) and electrolytic cells. In a galvanic cell, the redox reaction is spontaneous and produces electrical energy. In an electrolytic cell, an external power source is used to drive a non-spontaneous redox reaction in the opposite direction, consuming electrical energy to produce chemical energy.
Electrochemical cells have many practical applications, including batteries, fuel cells, electroplating, and corrosion protection.