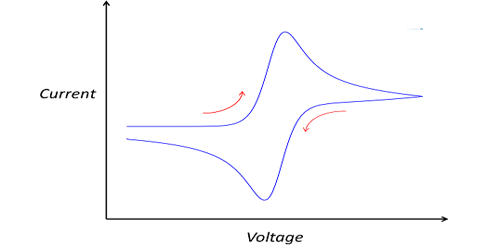
Cyclic voltammetry (CV) is a type of potentiodynamic electrochemical measurement. It is an electrochemical technique that measures the current that develops in an electrochemical cell under conditions where voltage is in excess of that predicted by the Nernst equation. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. This process is repeated multiple times during a scan and the changing current between the working and counter probes is measured by the device in real-time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode’s potential is ramped in the opposite direction to return to the initial potential. CV is a very powerful generic method for the electrochemical characterization of trace amounts of substances in water and deposits on conductive surfaces. The result is a characteristic duck-shaped plot known as a cyclic voltammogram.
Theory – “Cyclic voltammetry is a sophisticated potentiometric and voltammetric method. During a scan, the applied potential causes the chemical to be tested to undergo either oxidation (it loses an electron) or reduction (it gains an electron) depending on the direction of the ramping potential.”
CV is performed by cycling the potential of a working electrode and measuring the resulting current. It is another method commonly used to characterize biofilm growth and the effects of biocorrosion. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage (that is, the working electrode’s potential) to give the cyclic voltammogram trace. In these methods, the potential of the working electrode is linearly ramped up and down cyclically while measuring the current at the working electrode. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.
Applications
- Cyclic voltammetry is an electrochemical technique for measuring the current response of a redox-active solution to a linearly cycled potential sweep between two or more set values.
- Cyclic Voltammetry can be used to study qualitative information about electrochemical processes under various conditions, such as the presence of intermediates in oxidation-reduction reactions, the reversibility of a reaction.
- It is a useful method for quickly determining information about the thermodynamics of redox processes, the energy levels of the analyte, and the kinetics of electronic-transfer reactions.
- CV can also be used to determine the electron stoichiometry of a system, the diffusion coefficient of an analyte, and the formal reduction potential, which can be used as an identification tool.