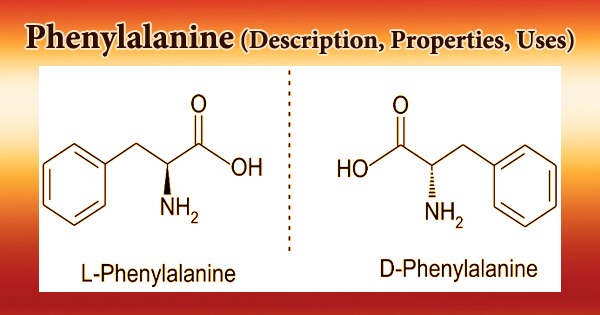
Phenylalanine (symbol Phe or F) is an essential aromatic amino acid in humans (supplied by food). It is crucial in the structure and function of numerous proteins and enzymes. It has the formula C9H11NO2 and is an essential -amino acid. It can be thought of as a benzyl group substituting for alanine’s methyl group, or a phenyl group substituting for alanine’s terminal hydrogen. It does, however, have antidepressant, analgesic, and pharmacological action at the niacin receptor II.
The suppression of enkephalin breakdown by carboxypeptidase A appears to be the mechanism of action for its analgesic effect. Because of the inert and hydrophobic nature of the benzyl side chain, this important amino acid is classed as neutral and nonpolar. The L-isomer is utilized in the biochemical synthesis of proteins that are coded by DNA. The amino acid phenylalanine is transformed to tyrosine, which is needed in the production of the neurotransmitters dopamine and norepinephrine.
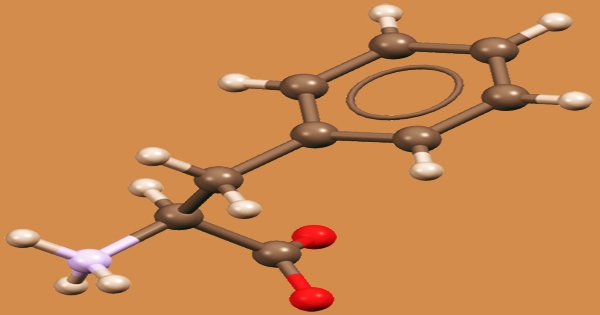
The L-form of Phenylalanine is used to make proteins, while the D-form is used to relieve pain. Phenylalanine absorbs UV radiation and is used to measure protein levels. It’s also employed as a pharmaceutical intermediate in the treatment and prevention of osteoporosis, cardiovascular disease, diabetes, and arteriosclerosis. By breaking the hydrogen bond between the active site base, Glu-270, and the zinc-bound water molecule, D-phenylalanine binding to carboxypeptidase A provides anion sensitivity to the enzyme’s function.
J. Heinrich Matthaei and Marshall W. Nirenberg found the genetic codon for phenylalanine in 1961. This discovery contributed to the understanding of the coding linkage that connects information stored in genomic nucleic acid with protein expression in live cells. The L-enantiomer of phenylalanine is L-phenylalanine. It functions as a nutraceutical, a vitamin, an Escherichia coli metabolite, a Saccharomyces cerevisiae metabolite, a plant metabolite, an algae metabolite, a mouse metabolite, a human xenobiotic metabolite, and an EC 3.1.3.1 (alkaline phosphatase) inhibitor, as well as a mouse metabolite.
The putative blockade of enkephalin breakdown by the enzyme carboxypeptidase A by D-phenylalanine could explain the alleged analgesic activity of DL-phenylalanine. Eggs, poultry, liver, beef, milk, and soybeans are all good sources of phenylalanine. Anything sweetened with the artificial sweetener aspartame, such as diet drinks, diet meals, and medication, is another major source of phenylalanine; aspartame metabolism creates phenylalanine as one of the compound’s metabolites.
It is a proteinogenic amino acid, a phenylalanine, and a L-alpha-amino acid from the erythrose 4-phosphate/phosphoenolpyruvate family. It’s an L-phenylalaninium conjugate base; it’s an L-phenylalaninate conjugate acid. It’s a tautomer of an L-phenylalanine zwitterion; it’s an enantiomer of a D-phenylalanine. The precursor role of L-phenylalanine in the creation of the neurotransmitters norepinephrine and dopamine could explain DL-alleged phenylalanine’s antidepressant action.
In 2002, the US Institute of Medicine’s Food and Nutrition Board (FNB) established Recommended Dietary Allowances (RDAs) for essential amino acids. Adults 19 years and older should take 33 mg/kg body weight per day of phenylalanine plus tyrosine. L-phenylalanine may benefit some people with depression, and it may also help with vitiligo therapy. It is biologically transformed to L-tyrosine, an amino acid that is also encoded by DNA.
L-tyrosine is then transformed to L-DOPA, which then becomes dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline). Catecholamines are the name for the last three. The small intestine absorbs D-phenylalanine, which is then delivered to the liver via the portal circulation. D-phenylalanine appears to be converted to L-phenylalanine in tiny amounts. L-phenylalanine appears to aggravate tardive dyskinesia in some schizophrenia patients and those who have used neuroleptic medications.
The beginning material for the production of flavonoids is phenylalanine. Lignan is made up of two amino acids: phenylalanine and tyrosine. The enzyme phenylalanine ammonia-lyase converts phenylalanine to cinnamic acid. Individual free amino acid concentrations in both the plasma and tissue pools can be significantly altered by dietary modifications or pathological circumstances. The artificial sweetener aspartame is a non-food source of phenylalanine. The body breaks down this molecule into many chemical byproducts, including phenylalanine.
Information Sources: