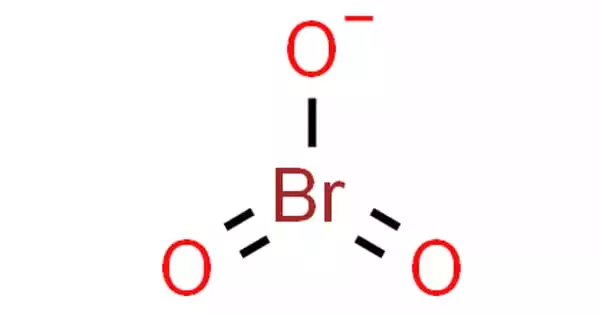
The bromate anion, BrO–3, is a bromine-based oxoanion. Bromates are an inorganic, colorless to light-colored solid. A bromate is a chemical compound that contains this ion. It is slightly soluble in water and denser than water. It is a bromine oxoanion and a monovalent inorganic anion. Bromates include sodium bromate (NaBrO3) and potassium bromate (KBrO3).
Bromates are formed many different ways in municipal drinking water. The most common is the reaction of ozone and bromide:
Br– + O3 → BrO–3
When bromide ion is present in the brine solution, electrochemical procedures such as the electrolysis of brine without a membrane to make hypochlorite will also produce bromate. Another example of an electrochemically inert system at bare electrodes is bromate reduction, which can be easily accomplished by homogenous reactants in the presence of catalysts (e.g., tungstates, molybdates). On fundamental principles, it is assumed that the reduction of BrO3 involves the creation of a complex with partially reduced tungstates or molybdates and that oxygen transfer to nonstoichimetric W oxie centers is simply a question of breaking the Br single bond O bond.
Photoactivation (sunlight exposure) will encourage liquid or gaseous bromine to generate bromate in bromide-containing water.
In laboratories bromates can be synthesized by dissolving Br2 in a concentrated solution of potassium hydroxide (KOH). The following reactions will take place (via the intermediate creation of hypobromite):
Br2 + 2 OH– → Br– + BrO– + H2O
3 BrO– → BrO–3 + 2 Br–

Bromate formation during ozonation
Although ozonation produces minimal byproducts, ozone combines with bromide ions in water to produce bromate. Bromide concentrations in freshwater can be high enough to create more than 10 ppb of bromate following ozonation—the maximum contamination threshold set by the USEPA. Lowering the pH of the water below 6.0, restricting ozone dosages, using an alternate water supply with a lower bromide concentration, pretreatment with ammonia, and adding modest amounts of chloramines prior to ozonation are all suggestions for reducing bromate formation.
Human health issues
Bromate in drinking water is undesirable since it is a possible carcinogen in humans. Its contact with the skin, eyes, and mucous membranes may cause irritation. It may be hazardous if consumed. Its presence in Coca-Dasani Cola’s bottled water prompted a recall in the United Kingdom. It is employed in the production of other compounds.
Iodate and bromate are environmental contaminants and suspected carcinogens that are generated, for example, in drinking water as a result of ozone treatment.