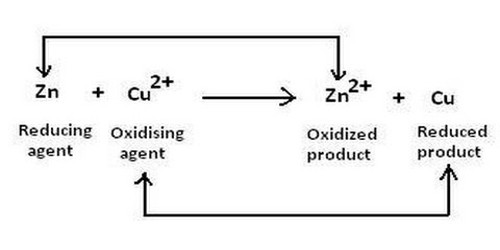
The oxidizing agent can have two meanings. It is a reactant that removes electrons from other reactants during a redox reaction. It means a reactant which oxidizes other element or reactant i.e., it undergoes reduce themselves and gain an electron. Hydrogen peroxide, ozone, oxygen, potassium nitrate, and nitric acid are all oxidizing agents. Ions, Atoms, and molecules having a strong affinity towards electrons are considered to be good oxidizers.
If one reagent in a reaction contributes oxygen, extracts hydrogen, or extracts electrons, it is said to be an oxidizing agent. It could be a chemical that releases oxygen atoms. It is a reactant that removes electrons from other reactants during a redox reaction. For example, potassium chlorate has a chemical formula of KClO3. When it oxidizes a reducing agent, such as powdered aluminum metal, it loses its oxygen to the aluminum and becomes potassium chloride, KCl. Almost any oxidizing agent might be expected to oxidize an organoborane, but few have received systematic investigation.
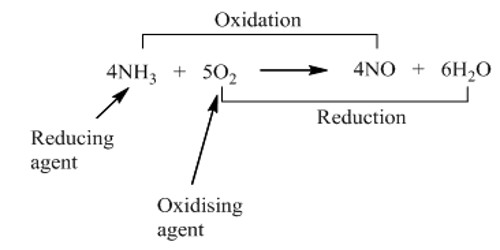
Another definition is a chemical that accepts electrons from a reducing agent. While an oxidizing agent gains electrons and is reduced in a chemical reaction, a reducing agent loses electrons and is oxidized during a chemical reaction. For example, potassium permanganate has an oxidation state of +7. In acid solution, it gains 5 electrons (e-), becoming a manganese compound with an oxidation state of +2. The stronger the electron affinity, the greater the oxidizing power. Most oxidizing agents of the second (electron-accepting) definition have oxygen, but not all. The reason why a substance is called an oxidizing agent is that it oxidizes another substance. For example, fluorine (F2), the most powerful oxidizing agent, does not have any oxygen in it. When an oxidizing agent gains electrons, it gets reduced, and, as a result, oxidizes the reducing agent. When it acts as an oxidizing agent, it gains an electron to transfer from an oxidation state of 0 to an oxidation state of -1. In a chemical reaction, whenever reduction takes place, oxidation also takes place.