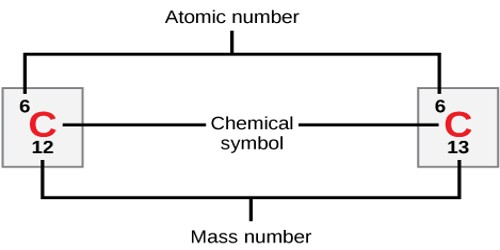
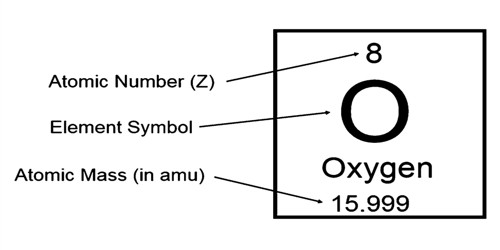
The atomic number (symbol: Z) of an atom is the number of protons in the nucleus of the atom. Atoms of each element contain a characteristic number of protons, the number of protons in an atom is called the atomic number. The atomic number of an atom identifies which element it is. For example, An atom of iron has 26 protons in its nucleus; therefore the atomic number of iron is 26. In a neutral atom, the atomic number is equal to the number of electrons orbiting the nucleus. The elements of the periodic table are listed in order of increasing atomic number.
Atomic number is not the same as:
- atomic mass (symbol: ma), which is the mass of a single atom, commonly expressed in unified atomic mass units
- mass number (symbol: A), which is the sum of the number of protons and number of neutrons in the nucleus of an atom
- relative atomic mass (also called atomic weight; symbol: Ar), which is the ratio of the average mass per atom of an element from a given sample to 1/12 the mass of a carbon-12 atom.
An atom can be classified as a particular element based solely on its atomic number. The atomic number of the periodic table directly corresponds to the number of protons which is in the atom. The periodic table displays all of the known elements and is arranged in order of increasing atomic number. Once another proton is added, it is no longer the same element. The same cannot be applied to when another neutron or another electron is added. An element’s or isotope’s atomic number tells how many protons are in its atoms.
The atomic number of an element never changes, meaning that the number of protons in the nucleus of every atom in an element is always the same. Electrons are the foundation for determining how compounds are formed amongst atoms. Adding or removing neutrons within an atom changes its isotope. An element’s or isotope’s mass number tells how many protons and neutrons in its atoms. As an example, carbon-12 is the most stable isotope for a carbon atom. However, we can add two more neutrons and carbon-12 is now carbon-14, a less stable isotope of carbon. The number of an isotope directly correlates to the atomic mass of an element. The amount of neutrons in any given atom by subtracting the atomic number from the atomic mass. For instance, iron, Fe, can exist in its neutral state, or in the +2 and +3 ionic states.