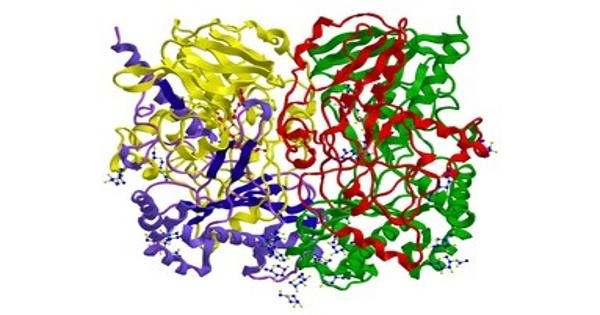
Oxidoreductases represent the largest class of enzymes in the Enzyme Commission nomenclature and comprise around one-third of all enzymatic activities registered in the Braunschweig Enzyme Database. In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. They are enzymes that catalyze the transfer of electrons from one molecule to another molecule. This group of enzymes usually utilizes NADP+ or NAD+ as cofactors. This class of enzymes catalyzes the oxidation of one chemical species with the concurrent reduction of another, facilitated by cofactors like NAD(P), flavin adenine dinucleotide (FAD), and flavin mononucleotide (FMN) as electron carriers.
Oxidoreductases are enzymes that catalyze oxidation/reduction reactions by transferring electrons, hydrogens, or oxygens from a reductant molecule to an oxidant molecule.
Oxidoreductases may help to promote a particular folding pathway both by selective disulfide catalysis and by acting as molecular chaperones that limit off-pathway folding choices. Transmembrane oxidoreductases create electron transport chains in bacteria, chloroplasts and mitochondria, including respiratory complexes I, II and III. Some others can associate with biological membranes as peripheral membrane proteins or be anchored to the membranes through a single transmembrane helix.
Reactions
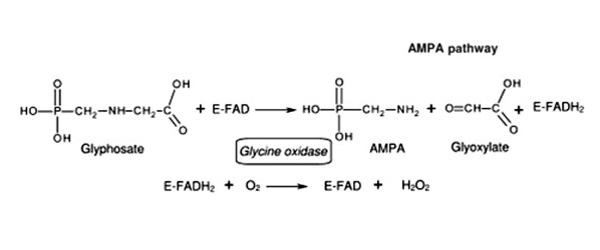
Oxidoreductases catalyze oxidation and reduction reactions that occur within the cell. For example, an enzyme that catalyzed this reaction would be an oxidoreductase:
A– + B → A + B–
In this example, A is the reductant (electron donor) and B is the oxidant (electron acceptor).
In biochemical reactions, the redox reactions are sometimes more difficult to see, such as this reaction from glycolysis:
Pi + glyceraldehyde-3-phosphate + NAD+ → NADH + H+ + 1,3-bisphosphoglycerate
In this reaction, NAD+ is the oxidant (electron acceptor), and glyceraldehyde-3-phosphate is the reductant (electron donor).
Oxidoreductases comprise of a large group of enzymes catalyzing the transfer of electrons from an electron donor to an electron acceptor molecule, commonly taking nicotinamide adenine dinucleotide phosphate (NADP) or nicotinamide adenine dinucleotide (NAD) as cofactors.
Function
Oxidoreductase enzymes play an important role in both aerobic and anaerobic metabolism. They can be found in glycolysis, TCA cycle, oxidative phosphorylation, and in amino acid metabolism. In glycolysis, the enzyme glyceraldehydes-3-phosphate dehydrogenase catalyzes the reduction of NAD+ to NADH. However, the re-oxidization of the generated NADH to NAD+ occurs in the oxidative phosphorylation pathway in order to maintain the redox state of the cell.
Information Source: