Heterogeneous catalysis is a type of catalysis where the catalyst occupies a different phase from the mixture of reactions. Phase distinguishes between not only components of solid, liquid and gas, but also immiscible mixtures (e.g., oil and water), or anywhere an interface occurs. Since the surface is that the place at which the reaction occurs, it generally is ready in ways in which produce large surface areas per unit of catalyst; finely divided metals, metal gauzes, metals incorporated into supporting matrices, and metallic films have all been utilized in modern heterogeneous catalysis. Catalysts are useful because they increase a reaction speed without being eaten by themselves, and are thus reusable.
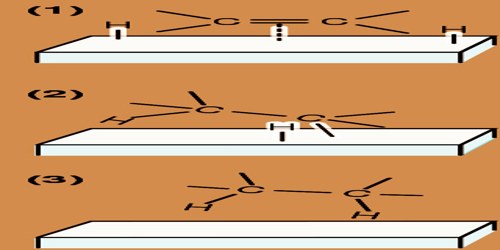
Actually, catalysts are chemical compounds that increase the speed of a reaction by lowering the energy of activation required to achieve the transition state. With solid catalysts, a minimum of one in each of the reactants is chemisorbed (a portmanteau term for chemically adsorbed) by the catalyst. Heterogeneous catalysis usually requires catalysts for the solid phase and reactants in the gas phase. During this case, there’s a cycle of molecular adsorption, reaction, and desorption occurring at the catalyst surface. Thermodynamics, mass transfer, and warmth transfer influence the speed (kinetics) of reaction.
Heterogeneous catalysis incorporates a number of advantages. For one, heterogeneous catalysts are often separated from a reaction mixture during a straightforward manner, like by filtration. During this way, an expensive catalyst is easily and effectively recovered, which is a vital consideration for industrial manufacturing processes. Heterogeneous catalysis is incredibly important because it enables faster, large-scale production and therefore the selective product formation.
Heterogeneous catalysis is predominantly the reliance of the chemical and energy industries. Thus, hydrogen is chemisorbed readily by many metals even at air temperatures (below −180 °C (−290 °F)). With a series of hydrogenation-dehydrogenation catalysts e.g., zinc oxide chromic oxide (ZnO–Cr2O3) chemisorption of hydrogen often occurs above temperature. Nitrogen is rapidly chemisorbed on synthetic ammonia-iron catalyst within the region above 400 °C (750 °F).
One drawback of heterogeneous catalysis however has to do with the catalyst’s available surface area. In heterogeneous catalysis, reactants diffuse to adsorb onto the catalyst surface from the bulk fluid phase. Once the catalyst’s surface is completely saturated with reactant molecules, the reaction cannot proceed until products leave the surface and some space is reopened to adsorb, or attach, a new reactant molecule. While the majority of heterogeneous catalysts are solids, there are a few practical-value variants. Other related isotopic studies have provided useful knowledge on the hydrocarbon reactions, using deuterium and carbon-14 as isotopic tracers.
Information Sources: