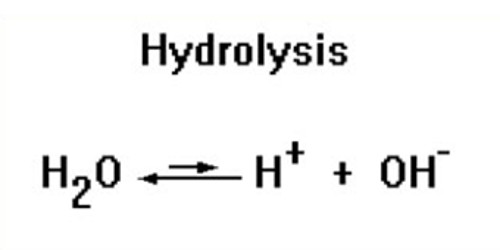
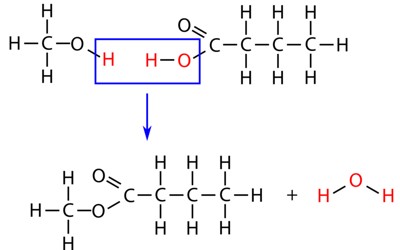
Hydrolysis is a chemical reaction or process where a chemical compound reacts with water. In practical terms, hydrolysis means the act of separating chemicals when water is added. This is the type of reaction that is used to break down polymers into many smaller units. It is the process variant where water reacts with a compound without causing its decomposition. In this reaction, water is always added to the chemical compound. There are three main types of hydrolysis: salt, acid, and base hydrolysis.
Hydrolysis of metal salts
Hydrolysis of metal salts is more commonly known as hydration. It occurs when salt from a weak base or acid dissolves in liquid. Many metal ions are strong Lewis acids, and in the water, they may undergo hydrolysis to form basic salts. Such salts contain a hydroxyl group that is directly bound to the metal ion in place of a water ligand. The positive charge on metal ions creates an attraction to water, a Lewis base with a non-binding electron pair on the oxygen atom, and alters the water molecule’s electron density. When this occurs, water spontaneously ionizes into hydroxide anions and hydronium cations.
This, in turn, increases the polarity of the O-H bond, which now acts as a proton donor under the Brønsted-Lowry acid-base theory to release the hydrogen as an H+ ion, increasing the acidity of the solution. For example, aluminum chloride undergoes extensive hydrolysis in water with the solution being very acidic.
[Al(H2O)6]3+ + H2O ↔ [Al(OH) (H2O)6]2+ + H3O+
This implies that hydrogen chloride is lost in the evaporation of AlCl3 solutions and the residue is a basic salt (in this case an oxychloride) in place of AlCl3. This involving ionic compounds may be illustrated by the chemical changes occurring in an aqueous solution of the salt sodium acetate. This type of reaction is also seen with other metal chlorides such as ZnCl2, SnCl2, FeCl3 and lanthanide halides such as DyCl3. With some compounds such as TiCl4, the hydrolysis may go to completion and form the pure hydroxide or oxide, in this case, TiO2. Hence, the solution exhibits basic properties (i.e., turns red litmus paper blue).