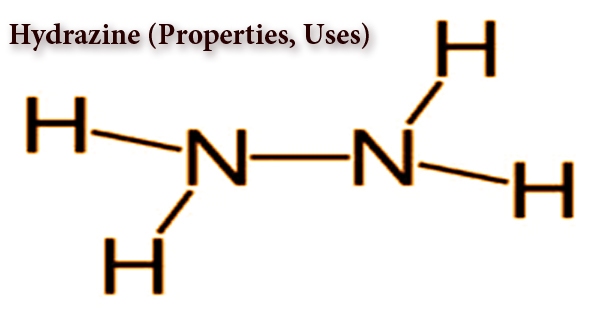
Polymers are large molecules made up of repeating subunits called monomers. These monomers are chemically bonded together through covalent bonds to form long chains or networks, which give the polymer its characteristic properties.
They’ve been called “dream” plastics: polyhydroxyalkanoates, or PHAs. They are a type of polymers that are naturally produced by living microbes or artificially synthesized from biorenewable feedstocks, and they are already the foundation of a young industry. They biodegrade in the natural environment, which includes soil and water.
However, there is a reason PHAs haven’t gained much traction as a sustainable, eco-friendly replacement for conventional plastics. Crystalline PHAs are brittle and therefore less practical and durable than traditional polymers. They are costly to make because they cannot be easily recycled and melt-processed.
Colorado State University polymer chemists led by Eugene Chen, University Distinguished Professor in the Department of Chemistry, have created a synthetic PHA platform that addresses each of these problems, paving the way for a future in which PHAs can take off in the marketplace as truly sustainable plastics.
We are adding three key desired features to the biological PHAs, including closed-loop chemical recycling, which is essential for achieving a circular PHA economy.
Eugene Chen
In the journal Science, Chen and colleagues describe a new class of modified PHAs that are easily accessible through chemical catalysis.
The intrinsic thermal instability of typical PHAs, which also makes it challenging to melt-process them into finished products, has the researchers looking for a solution.
The CSU scientists made significant structural alterations to these plastics by replacing the reactive hydrogen atoms that cause thermal breakdown with more durable methyl groups. The PHAs’ thermal stability is significantly improved by this structural change, resulting in plastics that may be melt-processed without decomposing.
Plus, these newly developed PHAs are mechanically stronger than the two most popular commercial plastics, isotactic propylene and high-density polyethylene, which are used to produce items like shampoo and milk bottles as well as synthetic fibers.
The best aspect is that the new PHA can be chemically broken down into its component monomer molecules using just a simple catalyst and heat. The recovered clean monomer may then be used to theoretically generate the same PHA indefinitely.
“We are adding three key desired features to the biological PHAs, including closed-loop chemical recycling, which is essential for achieving a circular PHA economy,” Chen said.