Carbonic Acid
Definition
Carbonic acid is a weak, unstable acid present in solutions of carbon dioxide in water. It gives carbonated beverages their sharp taste. Its chemical formula: H2CO3. Carbonic acid exists only in the form of its salts (carbonates), acid salts (hydrogen carbonates), amines (carbamic acid), and acid chlorides (carbonyl chloride). It is also a name sometimes given to solutions of carbon dioxide in water, which contain small amounts of H2CO3.

It is a weak acid that’s formed from the reaction of carbon dioxide dissolved in water. Let’s take a walk down memory lane and review the concept of weak acids. By definition, a weak acid is only partially ionized in a solution. In other words, weak acids don’t completely dissociate, or break apart, into ions in a solution.
Structure and Properties of Carbonic Acid
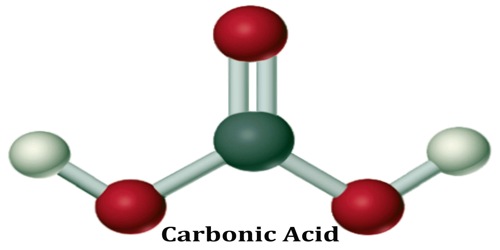
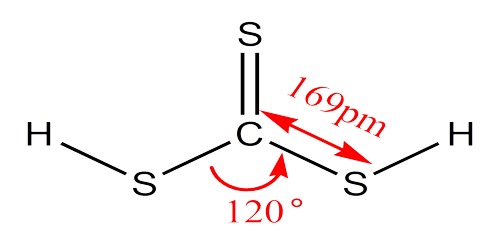
The chemical formula of carbonic acid is H2CO3. Its molecular formula is CH2O3, and its molar mass is 62.03 g/mol. The chemical structure of carbonic acid is shown below, and it consists of a carboxyl group, and two hydroxyl groups. It is a diprotic acid that can release two protons, but is only weakly acidic due to the strong O-H bonds.
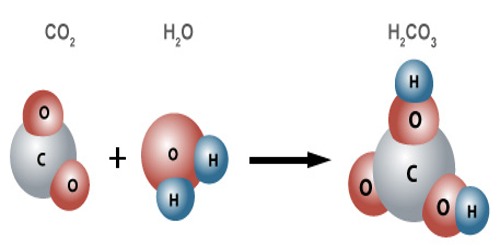
When carbon dioxide dissolves in water it exists in chemical equilibrium producing carbonic acid:
CO2 +H2O <=> H2CO3
The hydration equilibrium constant at 25 °C is called Kh, which in the case of carbonic acid is [H2CO3]/[CO2] ≈ 1.7×10−3 in pure water and ≈ 1.2×10−3 in seawater.
Industrially, carbonic acid is obtained as the by-product of other processes such as fermentation, fossil fuel burning, etc. Carbonic acid only exists as a solution, with a density of 1.668 g/mol. It is insoluble in water.
Carbonic acid is a weak and unstable acid, which partially dissociates in water into hydrogen ions (H+) and bicarbonate ions (HCO3–). Being a diprotic acid, it can form two kinds of salts, carbonates and bicarbonates. Addition of base to an excess of carbonic acid gives bicarbonate salts, while addition of excess base to carbonic acid gives carbonate salts.
Carbonic acid is a polyprotic acid, specifically it is diprotic meaning it has two protons which may dissociate from the parent molecule.
Health Effects (Role in Blood) of Carbonic Acid
Carbonic acid plays a very important role in mammalian blood. It is an intermediate during the transfer of carbon dioxide from the lungs to the blood and vice versa. The conversion of carbon dioxide into carbonic acid is catalyzed by an enzyme (carbonic anhydrase), which enhances the reaction rate by a factor of nearly a billion. Carbonic acid dissociates in the blood as in other solutions, to produce mainly H+ and HCO3– (bicarbonate) ions. This dissociation is an equilibrium reaction and it helps control the pH level of blood.
It is not considered toxic or hazardous, and is present in the human body. However, its exposure at high concentrations can irritate the eyes and respiratory tract.

Uses of Carbonic Acid
Carbonic acid is widely used in the preparation of bubbly drinks such as sodas, soft drinks, sparkling wines, and other aerated beverages. Carbonic acid is also used in many other fields, such as pharmaceuticals, cosmetics, fertilizers, food processing, anesthetics, etc.
Reference: