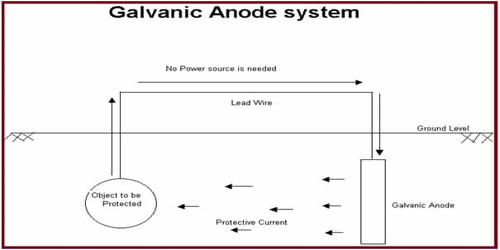
A galvanic anode is the main component of a galvanic cathodic protection (CP) system used to protect buried or submerged metal structures from corrosion. They are highly active metals that are used to prevent a less active material surface from corroding. In applications where the anodes are buried, a special backfill material surrounds the anode in order to ensure that the anode will produce the desired output.
They are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. They are made from a metal alloy with a more “active” voltage (more negative reduction potential/more positive electrochemical potential) than the metal of the structure. The difference in potential between the two metals means that the galvanic anode corrodes so that the anode material is consumed in preference to the structure. The current will flow from the newly introduced anode and the protected metal becomes cathodic creating a galvanic cell.
The loss (or sacrifice) of the anode material gives rise to the alternative name of a sacrificial anode. Sacrificial Anodes are used to protect the hulls of ships, water heaters, pipelines, distribution systems, above-ground tanks, underground tanks, and refineries.
Advantages and disadvantages of galvanic Anode protection
Advantages
- No external power sources required.
- Relatively easy to install.
- Lower voltages and currents mean that the risk of causing stray current interference on other structures is low.
- Require less frequent monitoring than impressed current CP systems.
- Relatively low risk of overprotection.
- Once installed, testing the system components is relatively simple for trained personnel.
Disadvantages
- Current capacity limited by anode mass and self-consumption at low current density.
- Lower driving voltage means the anodes may not work in high-resistivity environments.
- Often require that structure be electrically isolated from other structures and ground.
- Anodes are heavy and will increase water resistance on moving structures or pipe interiors.
- Where D.C. power is available, electrical energy can be obtained more cheaply than by galvanic anodes.
- Where large arrays are used wiring is needed due to high current flow and need to keep resistance losses low.
- Anodes must be carefully placed to avoid interfering with water flow into the propeller.
- To retain effectiveness, the anodes must be inspected and/or replaced as part of normal maintenance.
Cost-effectiveness
The Galvanic anodes are available in a variety of metals including magnesium, zinc, aluminum, and other alloys. As the anode materials used are generally more costly than iron, using this method to protect ferrous metal structures may not appear to be particularly cost-effective. These materials are available in various configurations with and without attached cables. However, consideration should also be given to the costs incurred to repair a corroded hull or to replace a steel pipeline or tank because their structural integrity has been compromised by corrosion.
However, there is a limit to the cost-effectiveness of a galvanic system. On larger structures or long pipelines, so many anodes may be needed that it would be more cost-effective to install impressed current cathodic protection.