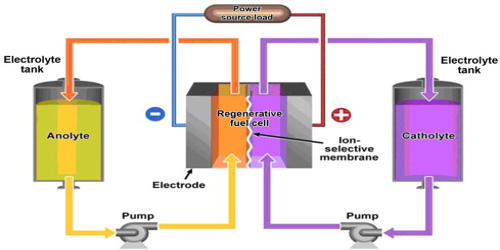
A flow battery, or redox flow battery, is an electrical storage device that is a cross between a conventional battery and a fuel cell. It is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids contained within the system and separated by a membrane. Ion exchange occurs through the membrane while both liquids circulate in their own respective space. It is a rechargeable battery in which electrolyte flows through one or more electrochemical cells from one or more tanks. The ion exchange that occurs between the cathode and anode generates electricity. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.2 volts. Flow batteries operate in a similar way to fuel cells and have been referred to as regenerative fuel cells.
A flow battery may be used as a fuel cell or as a rechargeable battery. The energy capacity is a function of the electrolyte volume, and the power is a function of the surface area of the electrodes. The electrochemical cells can be electrically connected in series or parallel, so determining the power of the flow battery system.
Construction principle
A flow battery is a rechargeable fuel cell in which an electrolyte containing one or more dissolved electroactive elements flows through an electrochemical cell that reversibly converts chemical energy directly to electricity. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; the electrodes are made of graphite bipolar plates. An additional electrolyte is stored externally, generally in tanks, and is usually pumped through the cell (or cells) of the reactor, although gravity feed systems are also known. Flow batteries can be rapidly “recharged” by replacing the electrolyte liquid while simultaneously recovering the spent material for re-energization. Flow batteries have been tried that contain precious metal, such as platinum, which is also used in fuel cells.
In other words, a flow battery is just like an electrochemical cell, with the exception that the ionic solution (electrolyte) is not stored in the cell around the electrodes. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power cell/reactor in which chemical energy is converted to electricity. Rather, the ionic solution is stored outside of the cell and can be fed into the cell in order to generate electricity. The total amount of electricity that can be generated depends on the size of the storage tanks.
Energy storage is becoming increasingly important to the power industry. Flow batteries are governed by the design principles established by electrochemical engineering. Usually, both the electroactive species in the redox pairs are soluble in aqueous acid or alkali solutions. However, in some flow batteries, such as zinc-bromine, one active species is deposited on the electrode.