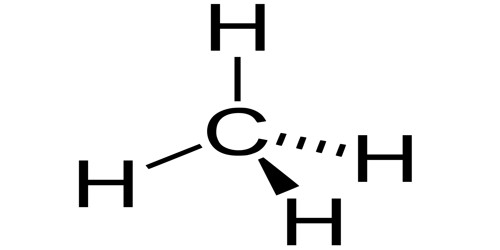
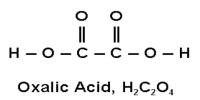Organic compounds are carbon-based compounds. They are defined as any compound whose molecules contain carbon and hydrogen or compound that is the derivative of it. Organic compounds contain carbon bonds in which at least one carbon atom is covalently linked to an atom of another type (usually hydrogen, oxygen or nitrogen). They are important because all living organisms contain carbon. Most polymers are organic compounds. They are the basic components of many of the cycles that drive the earth. Most of the foodstuffs that we consume every day such as sugar, fats, starch, vinegar, etc are basically organic compounds.
The term “organic compound” was coined by Berzelius in 1807.
General Characteristics –
- Can be isolated as well as prepared in the laboratory
- Comprise almost 90% of all known compounds.
- Mostly built up of only three elements- carbon, hydrogen, and oxygen.
- They are mostly insoluble in water but soluble in organic solvents.
- They are combustible in nature.
Types
- Carbohydrates – They are organic compounds made of the elements carbon, hydrogen, and oxygen. Examples: Glucose, Fructose, Sucrose, Cellulose, Glucose, etc.
- Lipids – They are made of carbon, hydrogen, and oxygen atoms. They have higher hydrogen to oxygen ratio than is found in carbohydrates. Examples: Cholesterol, Paraffin, Olive oil, Estrogen, etc.
- Proteins – They consist of chains of amino acids called peptides. Examples: Enzymes, Albumin, Hemoglobin, etc.
- Nucleic Acid – A nucleic acid is a type of biological polymer made up of chains of nucleotide monomers.
Kinds of organic compounds
There are natural organic compounds and synthetic ones. Their structure may be described by using names and making diagrams. Examples: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
- Natural Compounds
Natural compounds are compounds made by plants or animals. In the broadest sense, natural products include any substance produced by life. These could also be made in a lab, but many of these compounds are taken from nature. Common natural compounds are – amino acids, proteins, carbohydrates, many antibiotics like Penicillin and Amoxicillin.
- Synthetic compounds
Synthetic compounds are those made by people. It refers to a substance that is man-made by synthesis, rather than being produced by nature. Sometimes, this is done by taking something natural and changing the molecule in a small way, such as making glycerin from vegetable oils. Plastics are sometimes mostly natural, and other kinds are manufactured.
Structure
There are several ways of taking an unknown compound and finding out this structure:
- Mass spectrometry,
- X-ray diffraction,
- Nuclear magnetic resonance spectroscopy,
- Infrared spectroscopy.