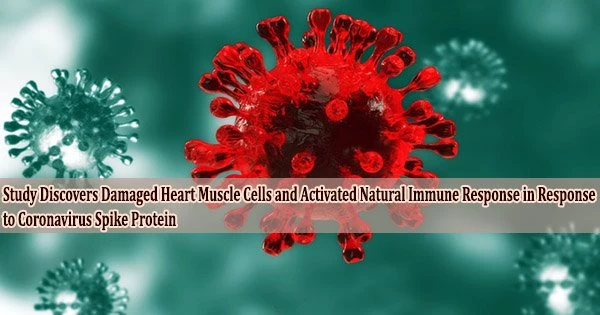
Many people are curious about how the virus affects the heart because cardiac damage is frequent in COVID-19 patients who are hospitalized.
According to preliminary research that will be presented at the American Heart Association’s Basic Cardiovascular Sciences Scientific Sessions in 2022, it has now been discovered that the spike protein from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus can cause heart muscle injury through the inflammatory process. The meeting, held in Chicago on July 25-28, offers the latest research on basic and translational cardiovascular science.
The SARS-CoV-2 virus, which is the source of COVID-19, has the spike protein on its surface. Angiotensin-converting enzyme 2 (ACE2) receptors on target cells are the point of attachment for spike proteins.
The first stage of infection is facilitated by the spike protein, which allows viruses to enter healthy cells. The virus can infect other organs in addition to the lungs, which can cause additional bodily harm, serious infection, and, in extreme cases, death.
“It’s already known from the clinical side that COVID-19 infection can induce heart injury, however, what we don’t know is the mechanistic details of how this occurs. What we suspect is that the spike protein has unknown pathological roles,” said Zhiqiang Lin, Ph.D., lead author of the study and an assistant professor at the Masonic Medical Research Institute in Utica, New York.
“Our data show that the spike protein from SARS-CoV-2 causes heart muscle damage. That’s why it’s important to get vaccinated and prevent this disease.”
“Host natural immunity is the first line of defense against pathogen invasion, and heart muscle cells have their own natural immune machinery. Activation of the body’s immune response is essential for fighting against virus infection; however, this may also impair heart muscle cell function and even lead to cell death and heart failure,” Lin said.
Our study provides two pieces of evidence that the SARS-CoV-2 spike protein does not need ACE2 to injure the heart. First, we found that the SARS-CoV-2 spike protein injured the heart of lab mice. Different from ACE2 in humans, ACE2 in mice does not interact with SARS-CoV-2 spike protein, therefore, SARS-CoV-2 spike protein did not injure the heart by directly disrupting ACE2 function. Second, although both the SARS-CoV-2 and NL63 coronaviruses use ACE2 as a receptor to infect cells, only the SARS-CoV-2 spike protein interacted with TLR4 and inflamed the heart muscle cells. Therefore, our study presents a novel, ACE2-independent pathological role of the SARS-CoV-2 spike protein.
Zhiqiang Lin
Researchers investigated whether heart muscle cells’ innate immune responses are triggered by the SARS-CoV-2 spike protein. HCoV-NL63 is a coronavirus that attacks the respiratory system without harming the heart, despite the fact that it similarly uses ACE2 to mediate virus entrance through its spike protein.
They investigated whether the NL63 spike protein and the SARS-CoV-2 spike protein may both lead to cardiac disease. Their findings demonstrated that, in contrast to the NL63 spike protein, the SARS-CoV-2 spike protein stimulated the body’s innate immune response in heart muscle cells and caused cardiac damage.
“The fact that the SARS-CoV-2 spike protein is activating the natural immune response may explain the high virulence compared to the other coronaviruses,” Lin said. “The TLR4 signaling is the major pathway that activates the body’s natural immune response, and the SARS-CoV-2 spike protein activates TLR4, not the regular flu spike protein.”
To investigate the impact of the SARS-CoV-2 spike protein on the heart, researchers cloned the SARS-CoV-2 spike protein and the NL63 spike protein into the AAV9 viral vector. Lab mice were given the AAV9 viral vector to activate the spike protein in the cardiac muscle cells. They discovered that heart dysfunction, hypertrophic remodeling (enlargement), and cardiac inflammation were caused by the SARS-CoV-2 spike protein as mediated by the AAV9 rather than the NL63 spike protein.
Researchers found that the SARS-CoV-2 spike protein significantly increased the size of cardiac muscle cells in laboratory experiments using grown human heart cells in dishes.
“We found direct evidence that the SARS-CoV-2 spike protein is toxic to heart muscle cells,” Lin said.
Researchers also looked at a cardiac biopsy from a deceased patient who had COVID-19-related inflammation. They found TLR4 and the SARS-CoV-2 spike protein in cardiac muscle cells as well as other cell types. On the other hand, a biopsy of a healthy human heart lacked these two proteins.
“That means once the heart is infected with SARS-CoV-2, it will activate the TLR4 signaling,” Lin said. “Besides directly damaging the heart muscle cells, the spike protein itself is very inflammatory and may cause systemic inflammation that indirectly causes heart problems.”
ACE2 is an important enzyme controlling blood pressure. The SARS-CoV-2 infection may affect ACE2 function, which raises blood pressure and damages the heart. SARS-CoV-2 may harm the heart through other, unidentified mechanisms.
“Our study provides two pieces of evidence that the SARS-CoV-2 spike protein does not need ACE2 to injure the heart. First, we found that the SARS-CoV-2 spike protein injured the heart of lab mice. Different from ACE2 in humans, ACE2 in mice does not interact with SARS-CoV-2 spike protein, therefore, SARS-CoV-2 spike protein did not injure the heart by directly disrupting ACE2 function. Second, although both the SARS-CoV-2 and NL63 coronaviruses use ACE2 as a receptor to infect cells, only the SARS-CoV-2 spike protein interacted with TLR4 and inflamed the heart muscle cells. Therefore, our study presents a novel, ACE2-independent pathological role of the SARS-CoV-2 spike protein,” Lin said.
This study is the first to investigate the potential cardiac effects of the SARS-CoV-2 spike protein. The next step for the researchers is to look into how SARS-CoV-2 spike proteins result in cardiac inflammation.
There are two possible mechanisms: the first is that the virus’ spike protein is expressed in infected heart muscle cells, directly triggering inflammation; the second is that the virus’ spike protein is shed into the bloodstream, where it causes heart damage from circulating SARS-CoV-2 spike proteins.
Co-authors are Caroline Sheldon, B.A.; Steven Negron, B.A.; Chase W. Kessinger, Ph.D.; Bing Xu, Ph.D.; William T. Pu, M.D.; and Chieh-Yu Lin, M.D., Ph.D. Authors’ disclosures are listed in the abstract. No funding was reported for this study.