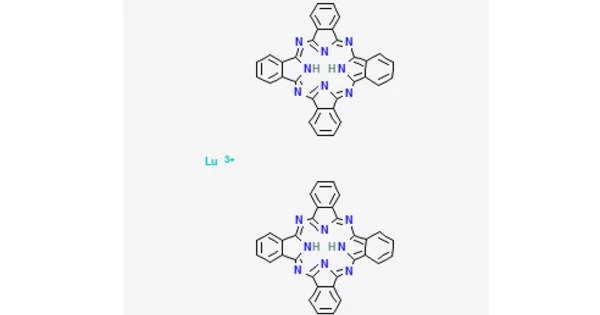
Lutetium phthalocyanine (LuPc2) is a coordination compound made up of lutetium and two phthalocyanines. It is a phthalocyanine compound. It was the first known example of a molecule that is an intrinsic semiconductor. When exposed to a voltage, it shows electrochromism, changing color. Due to their intriguing electrical properties, they are noted for their rich color and can be utilized as dyes, pigments, and in a variety of other applications.
Lutetium phthalocyanine, in particular, has lutetium (Lu) as the core metal atom that is coupled to a phthalocyanine ligand. LuPc2 is the chemical formula for lutetium phthalocyanine, where “Pc” stands for phthalocyanine. The coordination of the metal atom within the phthalocyanine ligand can have a substantial impact on the properties of the molecule, such as its electrical and optical properties.
Lutetium phthalocyanine and other comparable metal phthalocyanines have been investigated for use in organic electronics, solar devices, and catalysis. Because of their capacity to absorb light in the visible and near-infrared ranges, they are suited for use in photovoltaic cells and organic light-emitting diodes (OLEDs). In addition, the catalytic capabilities of metal phthalocyanines in chemical processes have been investigated.
Structure
LuPc2 is a double-decker sandwich chemical composed of a Lu3+ ion coupled to two phthalocyanine conjugate bases. The rings are staggered in their arrangement. The two ligands’ extremities are slightly deformed outwards. In the sense that the macrocycles carry an extra electron, the complex contains a non-innocent ligand. It is a free radical with an unpaired electron in a half-filled molecular orbital between the highest and lowest empty orbitals, allowing its electrical properties to be accurately controlled.
Properties
The properties and potential applications of lutetium phthalocyanine may vary depending on its specific synthesis and use, as well as the choice of other ligands and metals in the phthalocyanine structure.
LuPc2, as well as numerous substituted derivatives such as the alkoxy-methyl derivative Lu[(C8H17OCH2)8Pc]2, can be deposited as a thin film with intrinsic semiconductor characteristics due to its radical nature and low reduction potential when compared to other metal phthalocyanines. The oxidized form LuPc+2 is red, but the reduced form LuPc2 is blue, and the following two reduced forms are dark blue and violet, respectively. In aqueous solution containing dissolved alkali metal halides, the green/red oxidation cycle can be repeated over 10,000 times before being damaged by hydroxide ions; the green/blue redox degrades faster in water.
Electrical properties
LuPc2 and other lanthanide phthalocyanines are being researched for use in the fabrication of organic thin-film field-effect transistors. LuPc2 derivatives can be chosen to change color in the presence of specific molecules, such as in gas detectors; for example, in the presence of NADH, the thioether derivative Lu[(C6H13S)8Pc]2 changes from green to brownish-purple.