Acetate of strontium is a strontium compound. It is a white solid that, like other acetates, is water-soluble. It serves as a pathway for other chemicals such as barium acetate.
It is a compound used in some toothpaste to desensitize teeth to temperature extremes, sweets, acids, or contact. Desensitizing toothpaste are specifically designed to block the exposed areas of teeth that cause sensitivity. Tooth sensitivity, also known as “dentinal hypersensitivity,” is one of the most common dental complaints. This problem affects nearly one in every four adults and can begin as early as a person’s twenties.
Properties
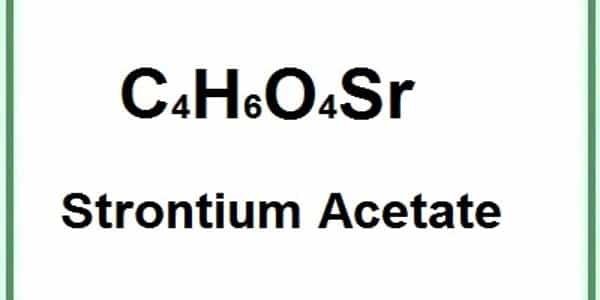
- Compound Formula: C4H6O4Sr
- Molecular Weight: 205.71
- Melting point: 150°C -0.5H₂O
- Density: 2,099 g/cm3
- Form: Powder
- Specific Gravity: 2.099
- Appearance/color: White to clear liquid
- Boiling Point: N/A
- Solubility in H2O: N/A
- Exact Mass: 205.932 g/mol
Strontium is an element that was discovered in 1790 and first isolated in 1808. It belongs to the group of alkaline-earth metals. It is a soft metal, similar to lead, with a silvery appearance when freshly cut. Pure metal does not exist in nature and can only be created through processing. It constitutes approximately 0.04 percent of the Earth’s crust and is most commonly found in the minerals strontianite (the carbonate) and celestite (the sulfate). It is used commercially as an ingredient in red signal flares.
The radioactive isotope strontium-90, with a half-life of 28 years, is regarded as the most dangerous component of nuclear fallout. It has the ability to replace some of the calcium in foods and eventually become incorporated into bones and teeth, where it continues to emit electrons and cause radiation injury. Radioactive strontium in controlled doses has been used to treat bone cancer.
Preparation
Strontium acetate is made by reacting strontium hydroxide or strontium carbonate with acetic acid. The acetate group can bind to metal ions in a variety of ways due to its two oxygen atoms, and several connectivities are observed for the various zinc acetate hydrates. Anhydrous zinc acetate has a polymeric structure composed of zinc coordinated to four oxygen atoms in a tetrahedral environment, with the acetate groups connecting each tetrahedron to its neighbors. Bidentate acetate ligands do not exist.
Production Methods: Strontium acetate, white crystals, soluble, formed by the reaction of strontium carbonate or hydroxide and acetic acid.
Uses
Strontium acetate is used in the manufacture of Laboratory reagents, Dental toothpaste, catalysts, chemical intermediates, medicines, etc.