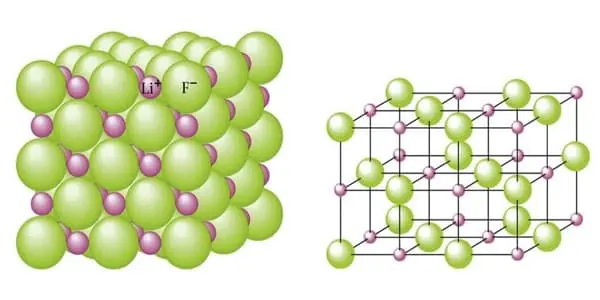
Lithium fluoride, also known as LiF, is an inorganic compound with the chemical formula LiF. It is a colorless solid that gradually turns white as the crystal size decreases. It is only slightly soluble in water, but it is soluble in HDF and other acids. It can, however, be cleaned with alcohol. Despite its lack of odor, lithium fluoride has a bitter-saline taste. It can be used to make rechargeable Li batteries, radiation dosimeters for personnel monitoring and radiation research, optical materials, heat sink materials, ceramics, and to dissolve fluid fuel in molten salt reactors.
It has a similar structure to sodium chloride, but it is much less soluble in water. It is primarily used as a constituent of molten salts. LiF formation from the elements produces one of the highest energies per mass of reactants, second only to BeO.
Properties
Lithium Fluoride (LiF) has the lowest refractive index of all common infrared materials. It possesses the highest UV transmission of any material, being able to transmit significantly into the VUV region at the hydrogen Lyman-alpha line (121nm).
- Molecular Weight: 25.94
- Appearance: White powder or transparent crystals
- Melting Point: 870 °C (1600 °F)
- Boiling Point: 1676 °C
- Density: 2.6 g/cm3
- Solubility in H2O: 0.27 at 293K
- Refractive Index: 1.4
- Crystal Phase / Structure: Cubic
Manufacturing
LiF is made by combining lithium hydroxide or lithium carbonate with hydrogen fluoride.
Lithium fluoride is a crystalline white solid. It is not hygroscopic like the other lithium halides and is unaffected by air exposure. The least soluble of the alkali metal fluorides is lithium fluoride. This property compares it to alkaline earth fluorides. Lithium fluoride, unlike the other lithium halides, does not form hydrates that can be isolated from solution. When hydrofluoric acid is added to an aqueous solution of lithium fluoride, its solubility increases. Under these conditions, the fluoride ion is converted to the bifluoride ion, HF-2, allowing the solid lithium fluoride to be dissolved further.
Preparation
Lithium fluoride is prepared by treating an aqueous solution of lithium hydroxide or lithium carbonate with aqueous hydrofluoric acid: LiOH + HF → LiF + H2O.
Applications
The most common application of lithium fluoride is as a flux in the manufacture of ceramics such as enamels, glasses, and glazes. It is also used in brazing and welding fluxes, as well as molten salt chemistry in metallurgy.
- Precursor to LiPF6 for batteries – Lithium fluoride reacts with hydrogen fluoride-HF and phosphorus pentachloride to form lithium hexafluorophosphate, an ingredient in lithium ion battery electrolyte.
- In molten salts – Fluorine is produced by electrolysis of molten potassium bifluoride in molten salts. When the electrolyte contains a few percent LiF, the electrolysis proceeds more quickly, possibly because it facilitates the formation of a Li-C-F interface on the carbon electrodes.
- Optics – Due to LiF’s large band gap, its crystals are more transparent to short wavelength ultraviolet radiation than any other material.
- Radiation detectors – It is also used in thermoluminescent dosimeters to record ionizing radiation exposure from gamma rays, beta particles, and neutrons.
- Nuclear reactors – The basic constituent of the preferred fluoride salt mixture used in liquid-fluoride nuclear reactors is lithium fluoride. Typically, lithium fluoride is combined with beryllium fluoride to form a base solvent (FLiBe), into which uranium and thorium fluorides are added.
- Cathode for PLED and OLEDs – It is widely used in PLED and OLED as a coupling layer to enhance electron injection. The thickness of the LiF layer is usually around 1 nm.
Toxicity
Lithium fluoride is a strong irritant to the eyes and skin; potassium bromide is toxic by ingestion and inhalation; and sodium chloride is table salt, which can cause medical problems if consumed in excess but poses no significant risk to emergency responders.