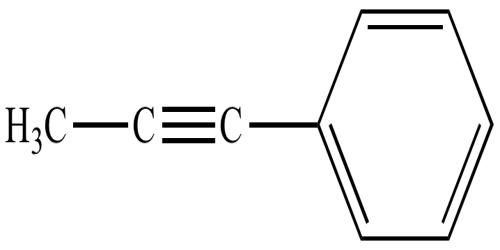
Alkyne is an unsaturated hydrocarbon containing at least one pair of triple-bonded carbons. It is any of the series of unsaturated hydrocarbons containing a triple bond, including acetylene. In organic chemistry, it is an unsaturated hydrocarbon containing at least one carbon-carbon triple bond. They comprise a series of carbon‐ and hydrogen‐based compounds that contain at least one triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. They are hydrocarbons, which are organic chemical compounds containing carbon (C) and hydrogen (H) atoms, and the feature that makes them recognized as alkynes is the presence of triple bonds. The simplest alkyne, ethyne (also known as acetylene), has two carbon atoms and the molecular formula of C 2H 2. The structural formula for ethyne is: H – C ≡ C- H.
Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Because alkynes have triple bonds in their chemical structure and consist of carbon and hydrogen atoms, they are unsaturated hydrocarbons. Like other hydrocarbons, alkynes are generally hydrophobic but tend to be more reactive.
Chemical properties
Alkynes are characteristically more unsaturated than alkenes. They can be prepared by elimination reactions under conditions similar to those used to form alkenes. Thus they add two equivalents of bromine whereas an alkene adds only one equivalent in a reaction with hydrobromic acid. Other reactions are listed below. In some reactions, alkynes are less reactive than alkenes. For example, in a molecule with an -ene and an -yne group, addition occurs preferentially at the -ene. They are unsaturated hydrocarbons. Like alkenes have the suffix –ene, alkynes use the ending –yne; this suffix is used when there is only one alkyne in the molecule.
Possible explanations involve the two π-bonds in the alkyne delocalizing, which would reduce the energy of the π-system or the stability of the intermediates during the reaction. Because an alkyne has two π bonds, two molar equivalents of HX must be eliminated from the starting material. They show a greater tendency to polymerize or oligomerize than alkenes do. The resulting polymers, called polyacetylenes (which do not contain alkyne units) are conjugated and can exhibit semiconducting properties.
Naming Alkynes
Like previously mentioned, the IUPAC rules are used for the naming of alkynes.
- Rule 1 – Find the longest carbon chain that includes both carbons of the triple bond.
- Rule 2 – Number the longest chain starting at the end closest to the triple bond. A 1-alkyne is referred to as a terminal alkyne and alkynes at any other position are called internal alkynes.
- Rule 3 – After numbering the longest chain with the lowest number assigned to the alkyne, label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order.
- Rule 4 – Substituents containing a triple bond are called alkynyl.
- Rule 5 – A molecule that contains both double and triple bonds is called an alkyne. The chain can be numbered starting with the end closest to the functional group that appears first.