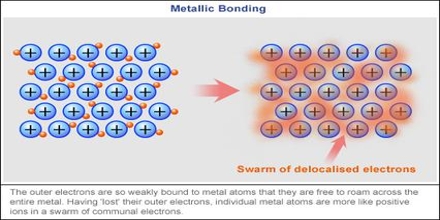
Metallic bonding is the strong attraction in between closely packed constructive metal ions plus a ‘sea’ of delocalised electrons. Atomic structure of a metal. The attraction between the metal ions and also the delocalised electrons should be overcome to melt or boil a steel. Metallic bonding is not the only form of chemical bonding any metal can exhibit, even as a pure substance.
Metallic Bonding