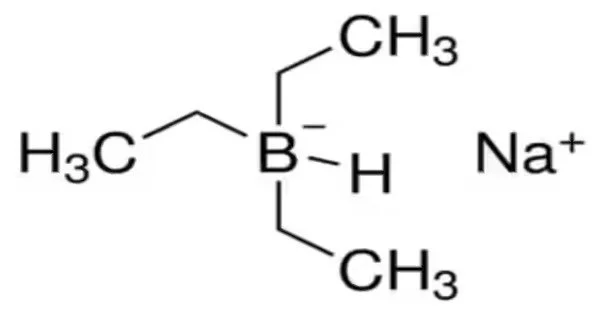
Sodium triethylborohydride is a reducing agent that is commonly used in organic chemistry for the reduction of various functional groups such as ketones, aldehydes, and esters. Its chemical formula is NaBH(OCH2CH3)3, and it is a white to light yellow powder that is soluble in polar solvents such as methanol and ethanol.
This reagent is considered to be milder than other borohydride reagents such as sodium borohydride, and it is commonly used in the synthesis of complex molecules. It is also used in pharmaceutical and agrochemical industries for the synthesis of various compounds.
It is an organoboron compound, unlike the related LiBH(C2H5)3, which is typically sold as a THF solution, it is a colorless, pyrophoric solid that is commercially available in toluene solution.
Properties
It is a white to off-white powder or crystalline solid. It is soluble in polar solvents such as alcohols, ethers, and dimethylformamide (DMF). It is a strong reducing agent that can reduce a variety of functional groups, including aldehydes, ketones, esters, and carboxylic acids. It is stable under normal conditions of use and storage, but it can decompose in the presence of water or other protic solvents.
- Chemical formula: C6H16BNa
- Molar mass: 121.99
- Appearance: white solid
- Melting point: 30 °C
- Molecular weight: 142.9 g/mol.
Application
It is regularly used in the reductive activation of homogeneous catalysts to convert metal halides to hydrides. Hot toluene slurry of sodium hydride was treated with triethylborane to produce sodium triethylborohydride. In toluene solution, the trimethylborohydride analogue, which is thought to be structurally similar to triethylborohydride, adopts a tetrameric structure.
However, it should be handled with care as it can react violently with water and some other common laboratory solvents, and it is highly flammable. Proper protective measures such as gloves, goggles, and a lab coat should be used when working with this reagent.
It should be stored under an inert atmosphere (such as argon or nitrogen) and in a dry place, away from moisture and oxidizing agents. It should be handled with care, as it is a flammable solid and can react violently with water or other protic solvents.