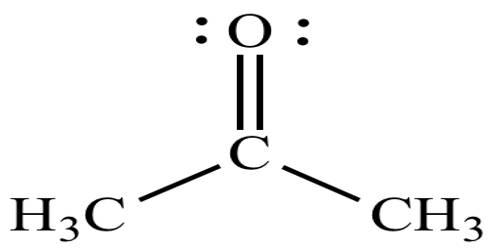
In chemistry, a ketone is a functional group with the structure RC(=O)R’, where R and R’ can be a variety of carbon-containing substituents. It is any of a class of organic compounds characterized by the presence of a carbonyl group in which the carbon atom is covalently bonded to an oxygen atom. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R’ = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in industry and in biology. They are found in several sugars and in compounds for medicinal use, including natural and synthetic steroid hormones. Examples include many sugars (ketoses), many steroids (e.g. testosterone), and the solvent acetone. Molecules of the anti-inflammatory agent cortisone contain three ketone groups.
Characterization
Ketones are highly reactive, although less so than aldehydes, to which they are closely related. An aldehyde differs from a ketone because of its hydrogen atom attached to its carbonyl group, making aldehydes easier to oxidize. Much of their chemical activity results from the nature of the carbonyl group. Ketones don’t have a hydrogen atom bonded to the carbonyl group and are more resistant to oxidation. They are structurally similar to aldehydes and also possess a carbonyl group. They are only oxidized by powerful oxidizing agents which have the ability to cleave carbon-carbon bonds. They are relatively stable compounds and are not easily oxidized further.
Qualitative organic tests
Ketones give positive results in Brady’s test, the reaction with 2,4-dinitrophenylhydrazine to give the corresponding hydrazone. Ketones may be distinguished from aldehydes by giving a negative result with Tollens’ reagent or with Fehling’s solution. A major reason is that the carbonyl group is highly polar; i.e., it has an uneven distribution of electrons. Methyl ketones give positive results for the iodoform test. Ketones also give positive results when treated with meta dinitrobenzene(m-dinitrobenzene) in the presence of dilute sodium hydroxide to give violet coloration. They can be produced by oxidation of secondary alcohols.
Applications
Ketones are produced on massive scales in the industry as solvents, polymer precursors, and pharmaceuticals. They can be synthesized by a wide variety of methods, and because of their ease of preparation, relative stability, and high reactivity, they are nearly ideal chemical intermediates. In terms of scale, the most important ketones are acetone, methylethylketone, and cyclohexanone. They are also common in biochemistry, but less so than in organic chemistry in general. They are most widely used as solvents, especially in industries manufacturing explosives, lacquers, paints, and textiles. The combustion of hydrocarbons is an uncontrolled oxidation process that gives ketones as well as many other types of compounds. Ketones are also used in tanning, as preservatives, and in hydraulic fluids.