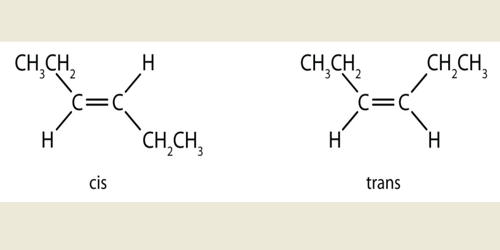
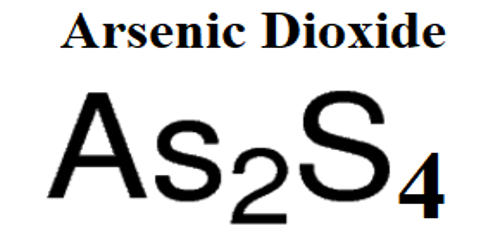Cis–trans isomerism, also known as configurational isomerism, is a term used in organic chemistry. These isomers occur where you have restricted rotation somewhere in a molecule. The prefixes “cis” and “trans” are from Latin: “this side of” and “the other side of”, respectively. At an introductory level in organic chemistry, examples usually just involve the carbon-carbon double bond – and that’s what this page will concentrate on. In the context of chemistry, cis indicates that the functional groups are on the same side of the carbon chain while trans conveys that functional groups are on opposing sides of the carbon chain. The identification of cis-trans isomers of unsaturated fatty acids cannot usually be achieved by GC-MS without reference substances.
Cis-trans isomers are stereoisomers, that is, pairs of molecules that have the same formula but whose functional groups are rotated into a different orientation in three-dimensional space. It is not to be confused with E–Z isomerism, which is an absolute stereochemical description. The E-Z system is better for naming more complicated structures but is more difficult to understand than cis-trans. In general, stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented. The simplest forms of stereoisomers are cis and trans isomers, both of which are created by the restricted rotation about a double bond or ring system.
Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis isomer is an isomer where two of the same atoms are on the same side of the double bond in a molecule. A trans-isomer consists of the molecule with two same atoms but here the atoms lie on the opposite side of the double bond. Trans and cis isomers have the same molecular formula and molecular weight, but they differ in some aspects. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms “syn” and “anti” are used. Cis-trans isomerism is of practical importance through its influence on the properties of industrially important materials.
The term “geometric isomerism” is considered by IUPAC to be an obsolete synonym of “cis-trans isomerism”. In the field of organic chemistry, cis isomers contain functional groups on the same side of the carbon chain whereas the functional groups are on opposite sides in trans-isomers.