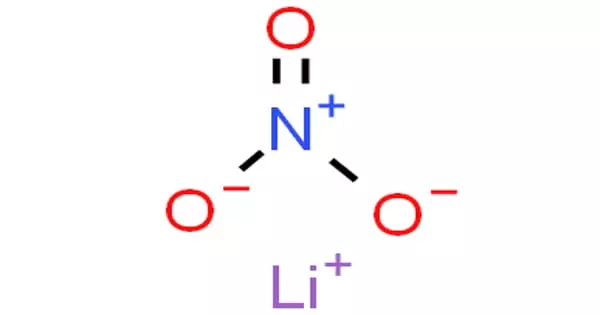
Lithium nitrate is an inorganic compound with the formula LiNO3. It has the appearance of a white to light yellow crystalline solid. It is the nitric acid lithium salt (an alkali metal nitrate). The salt is deliquescent, absorbing water to form lithium nitrate trihydrate. Its eutectics are appealing to heat transfer fluids. It is created by reacting nitric acid with lithium carbonate or lithium hydroxide. Contact with the material may cause skin, eye, and mucous membrane irritation.
This lithium salt is the result of a chemical reaction involving nitric acid and lithium carbonate. Water and carbon dioxide are two other byproducts of the chemical reaction.
Properties
Lithium nitrate is the inorganic nitrate salt of lithium. It has a role as an oxidizing agent. It is an inorganic nitrate salt and a lithium salt. All metallic nitrates are inorganic salts of a given metal cation and the nitrate anion. The nitrate anion is a univalent (-1 charge) polyatomic ion composed of a single nitrogen atom ionically bound to three oxygen atoms for a total formula weight of 62.05. Nitrate compounds are generally soluble in water.
- Melting point: 264 °C (lit.)
- Boiling point: 600 °C
- Density: 2.38
- Flash point: 600°C
- Storage temp. Store at +5°C to +30°C.
- Solubility H2O: 1 M at 20 °C, clear, colorless
- Form: Solid
- Color: White
- Specific Gravity: 2.38

Thermal storage
The hydrated form, lithium nitrate trihydrate, has a very high specific heat of fusion, 287±7 J/g, and can thus be used for thermal energy storage at its melt temperature of 303.3 K.
Lithium nitrate has been proposed as a medium for storing solar heat for cooking. A Fresnel lens would be used to melt solid lithium nitrate, which would then serve as a “solar battery,” allowing heat to be redistributed later through convection.
Preparation
Lithium nitrate is easily produced in the laboratory or in the industrial setting by reacting lithium hydroxide or lithium carbonate with nitric acid. To obtain anhydrous lithium nitrate, the resulting solution is evaporated and the solid is dried. Lithium nitrate dissolves readily in water. Moisture from the air is absorbed by the compound. It crystallizes from solution as lithium nitrate trihydrate below about 30 °C; above that temperature, anhydrous material precipitates, though the existence of a half-hydrate is not entirely settled.
To carry out the reaction, lithium hydroxide or lithium carbonate is added to the reactor containing distilled water, and nitric acid is slowly added while stirring and heating. Then we evaporate it to dryness and heat it under vacuum at around 200 ° C to obtain lithium nitrate.
HNO3 + LiOH → LiNO3 + H2O
Synthesis
Lithium nitrate can be synthesized by reacting nitric acid and lithium carbonate.
Li2CO3 + 2 HNO3 → 2 LiNO3 + H2O + CO2
A pH indicator is typically used when forming LiNO3 to determine when all of the acids has been neutralized. However, this neutralization can also be seen in the reduction of carbon dioxide production. The sample is heated to remove excess water from the final product.
Application
To increase the coulombic efficiency of lithium-sulfur batteries, lithium nitrate was added to the electrolyte solution of bis(trifluoromethane)sulfonimide. It can also be used to create spinel lithium titanate (Li4Ti5O12) materials via sol-gel synthesis.
This delectable colorless salt is an oxidizing agent used in the production of red fireworks and flares. Aside from industrial applications, nitrate lithium salt has chemical advantages. Lithium salt, for example, can be used as a heat exchange medium, a laboratory reagent, and an oxidizing agent.
Toxicity
When ingested, lithium nitrate can be toxic to the body, affecting the central nervous system, thyroids, kidneys, and cardio-vascular system. Lithium nitrate can irritate the skin, eyes, and mucous membranes when it comes into contact with them.