Any chemical compound with the empirical formula AlBrx is aluminium bromide. It is a light yellow colored chemical solution. It is a colorless, hygroscopic solid that can be sublimated. The most common form of aluminium bromide is aluminium tribromide.
In its anhydrous state, it appears as a white to yellowish-red lumpy solid with a pungent odor. It appears as a liquid in its aqueous form. Because it is a colorless, sublimable hygroscopic solid, old samples are usually hydrated, primarily as aluminium tribromide hexahydrate (AlBr3•6H2O). It is extremely damaging to the eyes, mucous membranes, and skin. It is widely used in the production of various chemicals.
Properties
Aluminum bromide, anhydrous appears as a white to yellowish-red, lumpy solid with a pungent odor. It appears as a light-yellow colored liquid.
- Chemical formula: AlBrx (hexahydrate)
- Molar mass: 266.694 g/mol (anhydrous); 374.785 g/mol (hexahydrate)
- Appearance: white to pale yellow powder
- Odor: pungent
- Density: 3.2 g/cm3 (anhydrous); 2.54 g/cm3 (hexahydrate)
- Melting point: 97.5 °C (anhydrous); 93 °C (hexahydrate)
- Boiling point: 255 (anhydrous)
- Solubility in water: very soluble, partially hydrolyses indicated by a fuming solution and an optional appearance of white precipitate
- Solubility: slightly soluble in methanol, diethyl ether, acetone
- Crystal structure: Monoclinic, mP16 (anhydrous)

Synthesis
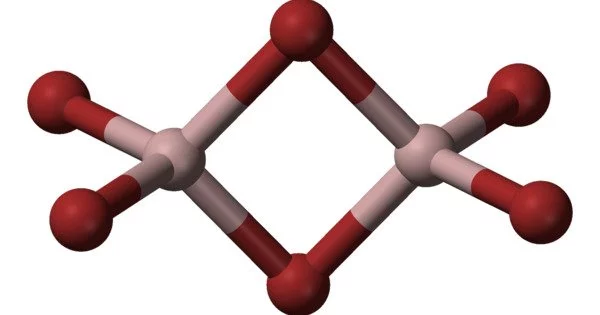
By far the most common form of aluminium bromide is Al2Br6. This species exists as hygroscopic colorless solid at standard conditions. Typical impure samples are yellowish or even red-brown due to the presence of iron-containing impurities. It is prepared by the reaction of HBr with Al:
2 Al + 6 HBr → Al2Br6 + 3 H2
Alternatively, the direct bromination occurs also:
2 Al + 3 Br2 → Al2Br6
Reactions
Al2Br6 readily dissociates to form the strong Lewis acid, AlBr3. In terms of the dimerization tendency of Al2Br6, it is common for heavier main group halides to exist as aggregates larger than implied by their empirical formulae. Because of the smaller size of the central atom, lighter main group halides, such as boron tribromide, do not exhibit this tendency.
Al2Br6 is hydrolyzed by water, resulting in the evolution of HBr and the formation of Al-OH-Br species, which is consistent with its Lewis acidic character. It also reacts quickly with alcohols and carboxylic acids, though not as vigorously as water. Al2Br6 forms adducts with simple Lewis bases (L), such as AlBr3L.
Aluminium tribromide reacts with carbon tetrachloride at 100 °C to form carbon tetrabromide:
4 AlBr3 + 3 CCl4 → 4 AlCl3 + 3 CBr4
and with phosgene yields carbonyl bromide and aluminium chlorobromide:
AlBr3 + COCl2 → COBr2 + AlCl2Br
Al2Br6 is used as a catalyst for the Friedel-Crafts alkylation reaction. Related Lewis acid-promoted reactions include as epoxide ring openings and decomplexation of dienes from iron carbonyls. It is a stronger Lewis acid than the more common Al2Cl6.
Safety
Aluminium tribromide is a highly reactive material.