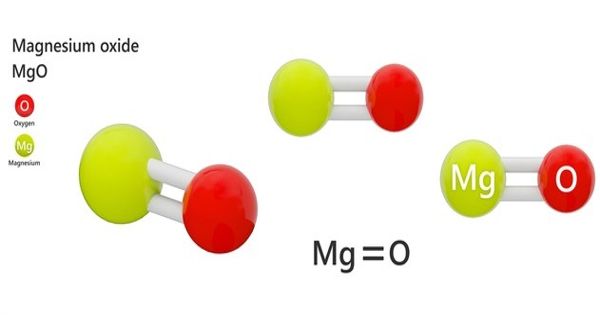
Magnesium oxide (MgO) is an inorganic salt of magnesium formed with ions of magnesium and oxygen. It is also known as, magnesia is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium. It is an inorganic salt of magnesium. It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2- ions held together by ionic bonding.
Magnesium hydroxide forms in the presence of water [MgO + H2O → Mg(OH)2], but it can be reversed by heating it to remove moisture. Even though it contains high amounts of magnesium, it has low absorbability in the body.
Properties
Magnesium oxide is an inorganic compound that occurs in nature as the mineral periclase. It was historically known as magnesia alba (literally, the white mineral from Magnesia – other sources give magnesia alba as MgCO3), to differentiate it from magnesia Negra, a black mineral containing what is now known as manganese.
- Molecular Weight: 40.3
- Appearance: White Powder
- Melting Point: 2,852° C (5,166° F)
- Boiling Point: 3,600° C (6,512° F)
- Density: 3.58 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 39.98
- Monoisotopic Mass: 39.98

Production
The majority of magnesium oxide produced today is obtained from the calcination of naturally occurring minerals. It is produced by the calcination of magnesium carbonate or magnesium hydroxide. The latter is obtained by the treatment of magnesium chloride MgCl2 solutions, typically seawater, with limewater or milk of lime.
Mg2+ + Ca(OH)2 → Mg(OH)2 + Ca2+
Calcining at different temperatures produces magnesium oxide of different reactivity. High temperatures 1500 – 2000 °C diminish the available surface area and produces dead-burned (often called dead burnt) magnesia, an unreactive form used as a refractory. Calcining temperatures 1000 – 1500 °C produce hard-burned magnesia, which has limited reactivity and calcining at a lower temperature, (700–1000 °C) produces light-burned magnesia, a reactive form, also known as caustic calcined magnesia.
Other important sources of magnesium oxide are seawater, underground deposits of brine, and deep salt beds where magnesium hydroxide [Mg(OH)2] is processed.
Uses
- It’s one of many forms of magnesium available for purchase in supplement form. It is used as a supplement to maintain adequate magnesium in the body.
- It’s added to dietary supplements as well as over-the-counter medications used to treat constipation, indigestion, and headaches. When used regularly, magnesium oxide can help boost low magnesium levels, relieve constipation, manage depression, treat migraines, and more.
- It is widely used in the steel industry as refractory brick. Magnesia bricks often in combination with spinel or chrome are also used in ferroalloy, non-ferrous, glass, and cement industries.
- It is widely used as a filling for electrical heating elements for applications in contact with air or liquids.
Information Source: