Sodium silicate is typically made by heating sodium carbonate (soda ash) and silicon dioxide (silica). It is the name of an organosilicon compound that contains the siloxide anion [(CH3)3C]3SiO−. Complexes of this large anionic ligand frequently adopt low coordination numbers. It is available in a variety of forms, including solid glass-like lumps and a liquid solution. Ti(silox)3, Nb(silox)3(PMe3), and [Cr(silox)3]− are a few examples.
Sodium silicate’s sodium oxide (Na2O) to silicon dioxide (SiO2) ratio can vary, resulting in different properties and applications.
Properties of silox and related complexes
Sodium silicate is a colorless, viscous liquid that is widely available. Depending on the concentration and method of preparation, it can also be obtained as a white powder or as a solid. It is extremely soluble in water. Depending on the concentration, it dissolves in water to form a clear to slightly cloudy solution.
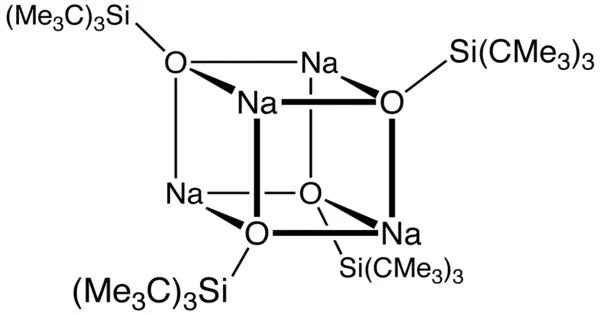
The sodium derivative, which has salt-like properties, is a cubane-type cluster. Related bulky anionic ligands include C5Me5−, (Me3Si)2N−, and NacNac−. Silox has a larger cone angle than C5Me5− and is a poorer donor. It is also larger than silylamides. For these reasons, silox anion forms highly unsaturated complexes.
Complexes with high coordinative unsaturation display high reactivity so long as they do not undergo intramolecular reactions. Silox offers this possibility. Examples of high reactivity exhibited by metal-silox compounds include the C-O bond in carbon monoxide and the C-N bond in pyridine.
Sodium silicate solutions are alkaline in nature, with a pH value typically ranging from 11 to 12.
Preparation
Na(silox) is typically formed by deprotonation of the silanol (t-Bu)3SiOH, which is formed through a multistep process involving the intermediacy of (t-Bu)2SiF2. It can also be produced by treating tBu3SiNa with nitrous oxide.
It is a versatile compound that undergoes a variety of chemical reactions. When combined with acids, it can form silica gel or solid silica. In addition, it can be used as a binder, catalyst, or corrosion inhibitor in a variety of applications.
Application
- Adhesive and binder: It is used in the manufacturing of various products like cardboard, paper, and fiber drums.
- Detergents and soaps: It is an ingredient in detergents and soaps, contributing to their cleaning and stain-removing properties.
- Cements and construction materials: It is used as a binding agent in cement and in the production of concrete and refractory materials.
- Water treatment: It can be used for water treatment, particularly for corrosion control and reducing scale formation.
- Automotive and industrial applications: It finds application in coatings, foundry molds, metal treatment, and as a component in fire-resistant materials.