Sodium peroxide (Na2O2) is a whitish solid (yellow when hot) that dissolves in ice water and decomposes in warm water or alcohol; r.d. 2.80; decomposes at 460°C. Crystallization of ice-water yields a crystalline octahydrate (hexagonal). This yellowish solid is the product of sodium being ignited in an environment of too much oxygen It’s a solid foundation. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and Na2O2·8H2O. The compound is created when sodium metal is burned in an environment with too much oxygen. It reacts with water at room temperature to produce sodium hydroxide and hydrogen peroxide.
In comparison to the anhydrous content, the octahydrate is white and easy to make. It is a potent oxidizing agent that produces iodate and periodate when it reacts with iodine vapour, carbonate when heated to 300°C, and nitrate when it reacts with nitrogen(II) oxide. It’s used as a bleaching agent in the manufacturing of wool and yarn, as well as in the refining of oils and fats and the manufacture of wood pulp. Friction, flame, or contact with moisture may easily ignite mixtures containing combustible material. When exposed to heat for an extended period of time, it can decompose rapidly, causing the containers to burst.
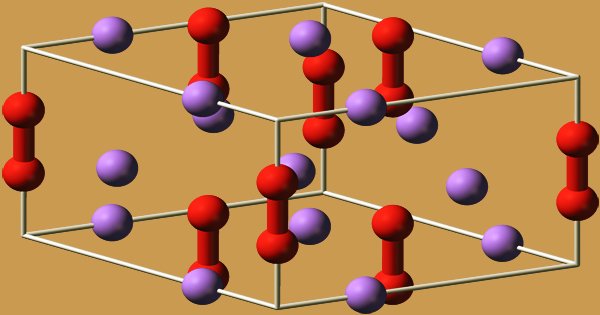
The hexagonal symmetry of sodium peroxide crystallizes. At 512 °C, the hexagonal form undergoes a phase transition into an unknown symmetry phase. Because of MMFF94’s unsupported part, mixture, or salt, conformer generation is not permitted. The compound decomposes to Na2O, releasing O2, when heated above the boiling point of 657 °C.
2 Na2O2 → 2 Na2O + O2
Sodium peroxide (Na2O2) is a pale-yellow solid that is hygroscopic and stable at room temperature. At about 300 °C, it begins to liberate oxygen and decomposes rapidly above its melting point of 460 °C. Sodium hydroxide is treated with hydrogen peroxide to create the octahydrate. The reaction of metallic sodium with oxygen at 130–200 °C, which produces sodium oxide, which absorbs oxygen in a separate step, can be used to make sodium peroxide on a large scale:
4 Na + O2 → 2 Na2O
2 Na2O + O2 → 2 Na2O2
Sodium peroxide (Na202) is a flammable white powder that turns yellow when heated and ignites when it comes into contact with water. Heating is needed to decompose it, which is not an easy task. It is stable in dry air, but decomposes quickly in moist air or when exposed to water. It can also be made by moving ozone gas through a platinum or palladium tube containing solid sodium iodide. The sodium is oxidized by the ozone, resulting in sodium peroxide. Iodine can be sublimed with a little heat. The reaction is catalyzed by platinum or palladium, which is unaffected by sodium peroxide.
Incandescence is generated when sodium peroxide and aluminum powder are combined. Spontaneous combustion happens when the mixture is exposed to moist air. According to the reaction, sodium peroxide hydrolyzes to give sodium hydroxide and hydrogen peroxide
Na2O2 + 2 H2O → 2 NaOH + H2O2
Sodium peroxide, depending on the circumstances, may be a powerful oxidizer or a powerful reducing agent. It’s also used as a bleach, in medicine soap, and to help minerals decompose. Spontaneous combustion occurs when a mixture of sodium peroxide and magnesium powder is exposed to moist air. Antimony, arsenic, copper, potassium, tin, and zinc are all oxidized to incandescence by sodium peroxide. In contact with water, alcohols, acids, powdered metals, and organic materials, a dangerous fire and explosion risk exists.
For the manufacture of paper and textiles, sodium peroxide was used to bleach wood pulp. It is currently primarily used for advanced laboratory activities, such as mineral extraction from different ores. Solozone and Flocool are two brand names for sodium peroxide. Bleaching animal and vegetable fabrics, feathers, bones, ivory, wood, wax, sponges, coral; making CO2-charged air respirable in torpedo vessels, submarines, diving bells, and other enclosed spaces; purifying air in sick rooms; dyeing and printing textiles; chemical analysis
Sodium peroxide is an oxidizing agent and an oxygen source that reacts with carbon dioxide to produce oxygen and sodium carbonate; it is thus especially useful in scuba gear, submarines, and other similar applications. Sodium peroxide is used as an oxidizing agent in chemistry experiments. It can also be used to create oxygen and sodium carbonate by reacting it with carbon dioxide:
2 Na2O2 + 2 CO2 → 2 Na2CO3 + O2
When heated to redness, the mixture of sodium peroxide and magnesium powder explodes. A mixture of powdered magnesium and sodium peroxide explodes when carbon dioxide gas is passed over it. Irritating to the shins, eyes, and mucous membranes. The chemical reaction creates a dangerous fire hazard; a powerful oxidizer. If breathing is difficult, give oxygen. Clothing and shoes that have been infected should be removed and isolated.
When dry, contaminated clothing can pose a fire hazard. If skin or eyes come into contact with the material, flush them with running water for at least 20 minutes. As a result, sodium peroxide is especially useful in scuba equipment, submarines, and other similar applications. Lithium peroxide is used in a similar way.
Information Sources: