Chromium(III) oxide (or chromia) is one of the Earth’s 10 most prevalent chemicals. It has the formula Cr2O3 and is an inorganic chemical. It is one of the most common chromium oxides and is used as a pigment. When employed as a pigment, it is known as “chrome green,” but when it was initially discovered, it was known as “viridian.”
Color additives containing chromium oxide greens are exempt from certification and are permanently listed for cosmetic use. Eskolaite, a rare mineral, is found in nature. Chromium(III) oxide is a brittle ceramic pigment (even a 50 percent mix with a high borax frit will not even begin to melt it in a crucible). It is the metal chromium’s only stable oxide.
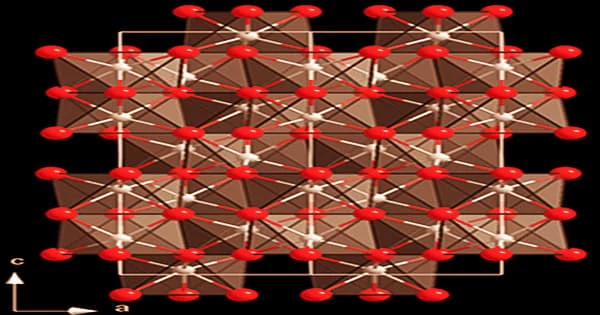
Cr2O3 has a corundum structure, which consists of a hexagonal close-packed array of oxide anions with chromium occupying 2/3 of the octahedral holes. It’s a crystalline powder that’s brilliant to dark green in color and insoluble in alkalis and acids. It’s made from chromite, a mineral found in southern Africa, Asia, Turkey, and Cuba.
Chrome, like other strong coloring additives, must be ground finely enough to avoid speckling in glass or glaze. Cr2O3 is a hard, brittle substance similar to corundum (Mohs hardness 8 to 8.5). Up to 307 K, the Néel temperature, it is antiferromagnetic. Acids have a hard time attacking it.
Chromium is a “fast” colorant, meaning it can produce vibrant green hues in any furnace condition, whether slow or fast, reducing or oxidizing. Because of its refractory nature, it is also a flat colorant that produces an army helmet opaque green.
Eskolaite, a mineral high in chromium, can be found in chromium-rich tremolite skarns, metaquartzites, and chlorite veins. Because chromium is so potent, only 2% of it is required to achieve a dark color. It’s not possible to make a metallic glaze with it.
Eskolaite is also an uncommon chondrite meteorite component. Pentti Eskola, a Finnish geologist, is honored with the mineral’s name. In raw glazes, chrome oxide is utilized, whereas in fritted glazes, potassium dichromate is used.
The process of converting chromite to chromia involves the reduction of Na2Cr2O7 with sulfur at high temperatures:
Na2Cr2O7 + S → Na2SO4 + Cr2O3
The oxide is also generated via the exothermic decomposition of ammonium dichromate or the decomposition of chromium salts such as chromium nitrate. Chromium oxide pigments, often known as chromium oxide green pigments, are made up of Cr2O3,Mr 151.99, chromium(III) oxide (1308-38-9).
Chromium oxide green is one of the few green-colored single-component pigments. Phosphorochrome green is a mixture of chrome yellow and blue phthalocyanine pigments; chrome green is a mixture of chrome yellow and blue phthalocyanine pigments.
Amphoteric chromium(III) oxide although it is insoluble in water, it interacts with acid to form hydrated chromium ions salts like (Cr(H2O)6)3+. It is also affected by concentrated alkali, resulting in the formation of (Cr(OH)6)3- salts.
It is converted to chromium metal when heated with finely divided carbon or aluminum:
Cr2O3 + 2 Al → 2 Cr + Al2O3
For the synthesis of chromium(III) oxide pigments, alkali dichromates are employed as starting materials. They are not categorized as hazardous materials and are therefore exempt from international transportation rules. Their use as a pigment is nearly limitless as long as they are kept dry.
Unlike traditional thermite reactions using iron oxides, chromium oxide thermite produces little or no sparks, smoke, or sound while glowing brightly. Casting chromium thermite is impossible due to the metal’s extremely high melting point.
Chromia was previously known as viridian and is widely used as a pigment due to its high stability. Chromium(III) oxide is a green pigment used to tint glass and fabrics. It is the colourant in “chrome green” and “institutional green.”
Metallurgy, as a component of refractory bricks, abrasives, and ceramics, and as a catalyst in hydrogenation, hydrogenolysis, and many other organic conversion reactions are all essential applications. Other chromium salts are also made with it.