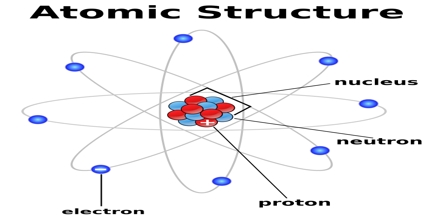
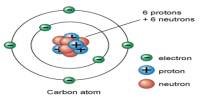
Principle purpose of this lecture is to describe on Atomic Structure. All elements are composed of particles called atoms. Atoms of one element can never be changed into another element. Here briefly describe on Dalton’s Atomic Theory. For explaining Atomic Structure, here focus on Thomson’s Electron Model and Rutherford’s Nuclear Model. Finally focus on what about the protons & neutrons and what makes atoms different from one another.
Atomic Structure