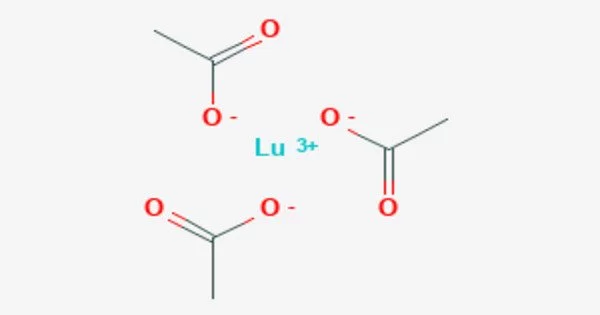
Lutetium acetylacetonate is a coordination molecule with the chemical formula Lu(C5H7O2)3, sometimes known as Lu(acac)3. Acetylacetonates are chelating ligands, which means they have several locations where they can connect to a central metal atom or ion. In the case of lutetium acetylacetonate, the core metal atom is lutetium (Lu). It is isomorphic to ytterbium acetylacetonate. It can be made by reacting trialkoxylutetium with acetylacetone.
These coordination compounds are employed in a variety of applications, including organic synthesis catalysts and materials. Lutetium acetylacetonate, in instance, may have features and applications unique to its combination of lutetium metal and acetylacetonate ligands.
Properties
- Appearance: It is typically a white to off-white powder or crystalline solid.
- Molecular Weight: The molar mass of lutetium acetylacetonate is approximately 581.34 g/mol.
- Solubility: It is generally soluble in organic solvents like acetone, toluene, and chloroform but has limited solubility in water.
- Melting Point: It has a relatively high melting point, typically above 200°C (392°F).
- Stability: It is stable under normal conditions but can decompose at elevated temperatures.
Magnetic Properties
Lutetium is a paramagnetic element, which means it has unpaired electrons and can be attracted by a magnetic field. This property may influence the magnetic behavior of lutetium acetylacetonate in certain situations.
Application
Lutetium acetylacetonate is frequently utilized in the synthesis of lutetium-based chemicals as well as in materials research and catalysis. It can be used to prepare thin films or nanoparticles for deposition. It is a common starting material in lutetium coordination chemistry, where the acetylacetonate ligands coordinate to the lutetium center.
Safety
When working with lutetium acetylacetonate, take the same precautions you would with any other chemical compound. It is critical to follow adequate safety requirements, such as wearing suitable protection equipment and operating in a well-ventilated location.