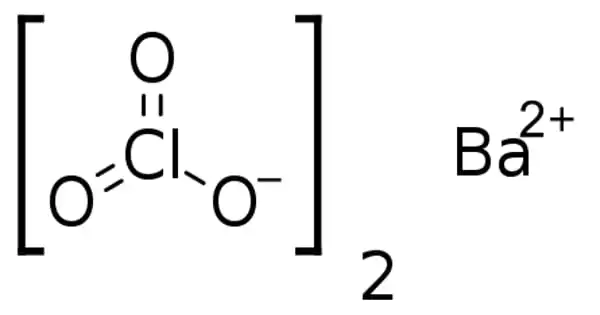The barium salt of chloric acid is barium chlorate. It is a white crystalline solid that is irritant and toxic, as are all soluble barium compounds. It is occasionally used in pyrotechnics to create a green color. It is also used in the manufacture of chloric acid. It is used in explosives and pyrotechnics, textile dyeing, and the production of other chlorates.
Properties
Barium chlorate has the appearance of a white crystalline solid. It combines with combustible materials to form highly flammable mixtures. If the combustible material is finely divided, the mixture may be ignited by friction and explosive.
- Molecular Weight: 322.26
- Appearance: white powder
- Melting Point: 414 °C
- Boiling Point: N/A
- Density: 3.18 g/cm3
- Solubility in H2O: N/A

Reactions
Synthesis
Barium chlorate can be produced through a double replacement reaction between solutions of barium chloride and sodium chlorate:
BaCl2 + 2 NaClO3 → Ba(ClO3)2 + 2 NaCl
When the resulting mixture is concentrated and chilled, barium chlorate precipitates. This is probably the most common preparation, taking advantage of barium chlorate’s lower solubility when compared to sodium chlorate.
The above method does result in some sodium contamination, which is undesirable for pyrotechnic purposes because sodium’s strong yellow can easily overpower barium’s green. Electrolysis can be used to produce sodium-free barium chlorate:
BaCl2 + 6 H2O → Ba(ClO3)2 + 6 H2
It can also be produced by the reaction of barium carbonate with boiling ammonium chlorate solution:
2 NH4ClO3 + BaCO3 + Q → Ba(ClO3)2 + 2 NH3 + H2O + CO2
The reaction initially produces barium chlorate and ammonium carbonate; boiling the solution decomposes the ammonium carbonate and drives off the resulting ammonia and carbon dioxide, leaving only barium chlorate in solution.
The green in this firework is caused by barium chlorate and barium nitrate.
Decomposition
When exposed to heat, barium chlorate alone will decompose to barium chloride and oxygen:
Ba(ClO3)2 → BaCl2 + 3 O2
Chloric acid
Through the reaction of barium chlorate with dilute sulfuric acid, which results in a solution of chloric acid and insoluble barium sulfate precipitate, chloric acid, the formal precursor to all chlorate salts, is produced:
Ba(ClO3)2 + H2SO4 → 2 HClO3 + BaSO4
Before mixing, both the chlorate and the acid should be prepared as dilute solutions so that the chloric acid produced is dilute, as concentrated solutions of chloric acid (above 30%) are unstable and prone to decompose, sometimes explosively.
Commercial uses
When barium chlorate is burned with fuel, it emits a bright green light. Because it is an oxidizer, a chlorine donor, and contains a metal, this compound produces an unrivaled green color. However, because all chlorates are unstable to sulfur, acids, and ammonium ions, they have been banned from use in class C fireworks in the United States. As a result, an increasing number of fireworks manufacturers have begun to use more stable compounds such as barium nitrate and barium carbonate.
Environmental Hazard
Humans are poisoned by barium chlorate, which is also harmful to the environment. If it is leached into bodies of water, it is extremely toxic to aquatic organisms. Although chemical spills of this compound are uncommon, they can harm entire ecosystems and should be avoided. This compound must be disposed of as hazardous waste. Barium chlorate is classified as hazardous by the Environmental Protection Agency (EPA).