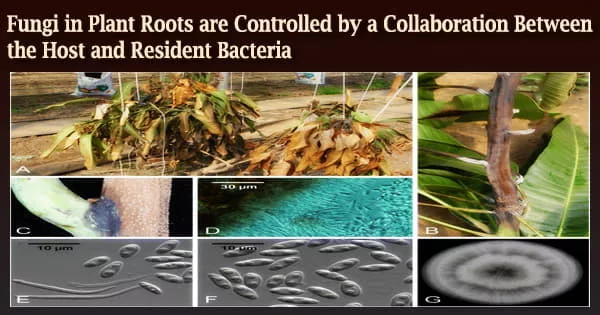
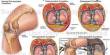
In nature, bacteria and filamentous eukaryotes (fungi and oomycetes) invade the roots of healthy plants, and the makeup of these communities has a significant impact on plant health.
Plants need to maintain a microbial equilibrium in their roots in order to stay healthy, but the mechanisms by which they do so are still largely unknown.
Stéphane Hacquard and colleagues from the MPIPZ’s Department of Plant-Microbe Interactions in Cologne, Germany, have now published a new study in PNAS that sheds light on the host and microbial factors that are required to maintain a beneficial relationship between plant roots and their diverse microbial partners.
To tackle this research question, the first author of the study Katarzyna W. Wolinska used a complex microbial community comprising 183 bacteria (B), 25 fungi (F), and 6 oomycetes (O) that were isolated from roots of healthy Arabidopsis thaliana (Thale Cress) plants. In comparison to sterile control plants produced in the absence of microorganisms, she discovered that this diverse BFO community aided plant growth.
The scientists then proposed that inactivating key components of the plant’s innate immune system, which is responsible for fighting pathogen invasion, would alter the microbial balance in roots, altering plant health.
The beneficial BFO community was no longer useful in numerous of the immunocompromised mutant plants, confirming this notion.
Inactivation of two plant host genes involved in tryptophan-derived specialized antimicrobials, in particular, was enough to transform the helpful BFO community into a harmful community that harmed plant performance.
The researchers then looked for aberrant microbial signatures in the roots of these immunocompromised plants and discovered that the fungal load in their roots was the most important element in explaining growth discrepancies amongst plants.
Our results illustrate how host- and bacterium-encoded functions act in concert to maintain fungi in check-in Arabidopsis roots, thereby promoting plant health and maintaining growth-promoting activities of multi-kingdom microbial communities.
Stéphane Hacquard
This result led to the conclusion that, in the absence of a functional immune system, the fungal burden detected in plant roots was most likely the primary cause of the shift from a healthy to a sick state.
K. W. Wolinska used the B, F, and O communities separately or in various combinations (BO, FO, BF, BFO) to further investigate whether the presence of fungi in the plant root microbial community was the direct cause of disease observed in the plants, and found that the presence of the fungi was indeed necessary to induce the unhealthy state of the plants.
These findings suggest that the host plant’s synthesis of particular anti-fungal compounds during tryptophan metabolism is critical for maintaining a healthy fungal balance in plant roots.
Even in the presence of a fully functional immune system, these anti-fungal compounds appeared to be insufficient in entirely defending plants from fungi in the absence of bacteria.
“Our results illustrate how host- and bacterium-encoded functions act in concert to maintain fungi in check-in Arabidopsis roots, thereby promoting plant health and maintaining growth-promoting activities of multi-kingdom microbial communities,” says study leader Stéphane Hacquard.
The discovery that the bacterial community’s protective action is just as crucial for regulating fungus as the host’s innate immune branch involving tryptophan-derived specialized chemicals is surprising.
It suggests that the plant immune system is insufficient to fully defend plants from fungal infection and that bacterial partners living in roots provide an additional layer of defense that is required for plant survival.
These findings could help to improve plant health by converting potentially hazardous fungus into beneficial isolates. It is now possible to create mixed bacterial-fungal synthetic communities that are projected to deliver considerable fitness benefits to the host using the knowledge gained in this work.