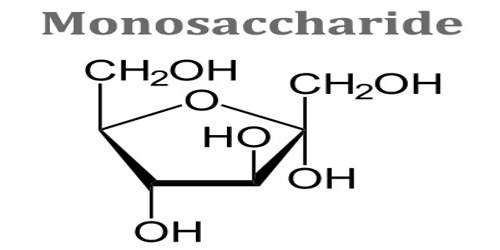
Monosaccharide
Definition
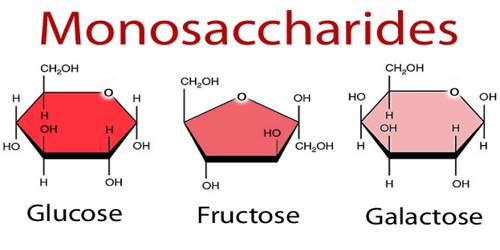
Monosaccharide is any of a class of carbohydrates that cannot be broken down to simpler sugars by hydrolysis and that constitute the building blocks of oligosaccharides and polysaccharides. The general formula is CnH2nOn. They are the simplest form of sugar and are usually colorless, water-soluble, and crystalline solids. Some monosaccharides have a sweet taste. It can occur as chains or rings. Fructose, glucose, and ribose are monosaccharides. It is also called simple sugar.
Carbohydrates are biological molecules that contain carbon (C), hydrogen (H), and oxygen (O) atoms. Carbohydrates are very important because they provide energy and fuel for our bodies so that our brains can function properly and so that our muscles can work. Carbohydrates are our preferred source of energy.
Monosaccharide naming further distinguishes the highest priority functional group and number of carbon atoms as well as the chirality of the highest numbered chiral carbon. The functional group determines the molecule’s prefix, while the suffix “ose” is applied generally to all molecules in this class of sugars.
Structure and Functions of Monosaccharide
All monosaccharides have the same general formula of (CH2O)n, which designates a central carbon molecule bonded to two hydrogens and one oxygen. The oxygen will also bond to a hydrogen, creating a hydroxyl group. Because carbon can form 4 bonds, several of these carbon molecules can bond together.
Glucose is one of the most common monosaccharides in nature, used by nearly every form of life. The most important monosaccharide, glucose, is a hexose. Examples of heptoses include the ketoses mannoheptulose and sedoheptulose. Monosaccharides with eight or more carbons are rarely observed as they are quite unstable. In aqueous solutions monosaccharides exist as rings if they have more than four carbons.
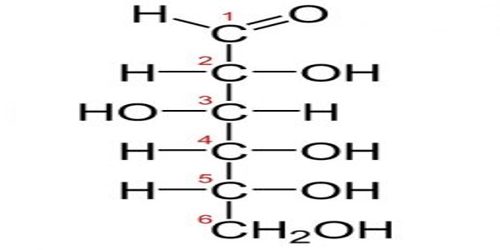
Typically, monosaccharides with more than 5 carbons exist as rings in solutions of water. The hydroxyl group on the fifth carbon will react with the first carbon. The hydroxyl group gives up its hydrogen atom when it forms a bond with the first carbon. The double bonded oxygen on the first carbon bonds with a new hydrogen when the second bond with the carbon is broken. This forms a fully connected and stable ring of carbons.
Monosaccharides are classified as well based on their functional groups. A functional group is categorized by atoms or bonds that are responsible for the chemical reactivity within a molecule. It can by combined through glycosidic bonds to form larger carbohydrates, known as oligosaccharides or polysaccharides. An oligosaccharide with only two monosaccharides is known as a disaccharide. When more than 20 monosaccharides are combined with glycosidic bonds, an oligosaccharide becomes a polysaccharide. Some polysaccharides, like cellulose, contain thousands of monosaccharides. A monosaccharide is a type of monomer, or molecule that can combine with like molecules to create a larger polymer. An aldehyde group is a carbon atom forming a double bond with oxygen and a single bond with hydrogen.
Reference: