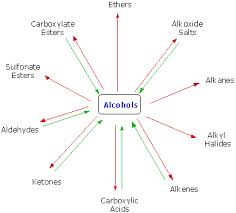
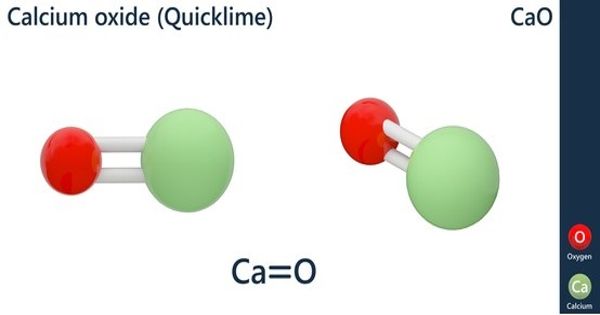
Basic objective of this article is to discuss on Reactions of Alcohols. Alcohols are equipped for being converted to metal salts, alkyl halides, esters, aldehydes, ketones, as well as carboxylic acids. Acidity of alcohols lessens while going from key to secondary to tertiary. This decrease in acidity is because of two factors: an increase of electron density around the oxygen atom of the more highly‐substituted alcohol, and steric hindrance. Both of these situations increase the activation energy for proton removal.
Discuss on Reactions of Alcohols