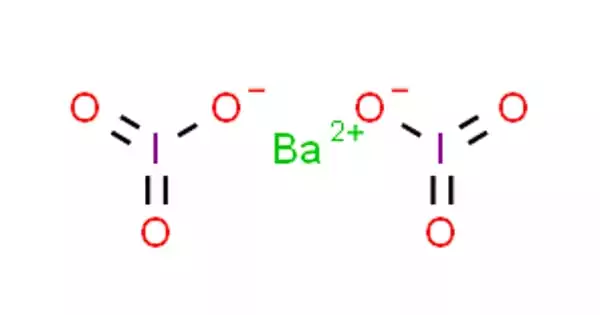
Barium iodate is an inorganic chemical compound with the chemical formula Ba(IO3)2. It is a white, granular substance. Barium is a silvery-white metal which exists in nature only in ores containing mixtures of elements. It combines with other chemicals such as sulfur or carbon and oxygen to form barium compounds.
Properties
The monohydrate occurs as lustrous, monoclinic crystals or a white crystalline powder. It is somewhat soluble in hot water but slightly in cold water. It is insoluble in sulfuric acid or alcohol. It loses its water of hydration at 130°C.The solubility product of the monohydrate at 25°C is 2.7×10-9. This salt is not used in Industry but is offered for sale commercially in limited quantities.
- Molecular Weight: 487.132
- Appearance: white crystals or powder
- Melting Point: decomposes at 476℃
- Boiling Point: N/A
- Density: 4.998 g/cm3
- Solubility in H2O: N/A

Derivation
Barium iodate can be derived either as a product of a reaction of iodine and barium hydroxide or by combining barium chlorate with potassium iodate. Solubility equilibrium is a type of heterogeneous equilibrium created in a saturated aqueous solution of a salt compound. It is heterogeneous because the reactant is the solid (undissolved) compound and the products are the constituent dissociated ions in solution.
Chemical properties
The compound is stable on a temperature up to approximately 580 °C (1,076 °F). If the temperature is higher than that value, the following reaction, known as Rammelsberg’s reaction, occurs:
5 Ba(IO3)2 → Ba5(IO6)2 + 9 O2 + 4 I2
Preparation
Barium Iodate can be prepared by the reaction of iodic acid upon the carbonate:
BaCO3 +2HIO3 → Ba(IO3)2 +CO2 +H2O
The product is either a monohydrate or a hexahydrate (like the calcium homologue).
It is somewhat soluble in hot water but slightly in cold water. It is insoluble in sulfuric acid or alcohol. It loses its water of hydration at 130°C.
Application
Barium compounds are used by the oil and gas industries to make drilling muds. Drilling muds make it easier to drill through rock by keeping the drill bit lubricated. They are also used to make paint, bricks, ceramics, glass, and rubber.
This salt is not used in Industry but is offered for sale commercially in limited quantities. The product is either a monohydrate or a hexahydrate (like the calcium homologue).