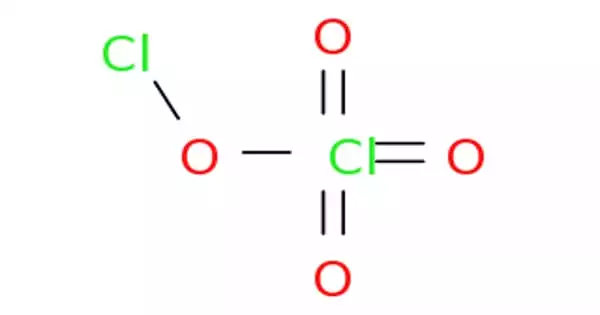
Chlorine perchlorate is a chemical compound with the formula Cl2O4. It is a chlorine oxide that is the chloro derivative of perchloric acid. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It derives from perchloric acid. It is produced by the photolysis of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light:
2 ClO2 → ClOClO3
Chlorine perchlorate can also be made by the following reaction at −45 °C.
CsClO4 + ClOSO2F → Cs(SO3)F + ClOClO3
Properties
- Molar mass: 134.90 g·mol−1
- Appearance: Pale green liquid
- Density: 1.81 g cm−3
- Melting point: −117 °C (−179 °F; 156 K)
- Boiling point: 20 °C (68 °F; 293 K) (decomposes)
Chlorine perchlorate is a pale greenish liquid. It is less stable than ClO2 (chlorine dioxide) and decomposes at room temperature to give O2 (oxygen), Cl2 (chlorine), and Cl2O6 (dichlorine hexoxide):
2 ClOClO3 → O2 + Cl2 + Cl2O6
Chlorine perchlorate reacts with metal chlorides to form chlorine and the corresponding anhydrous perchlorate:
CrO2Cl2 + 2 ClOClO3 → 2 Cl2 + CrO2(ClO4)2
TiCl4 + 4 ClOClO3 → 4 Cl2 + Ti(ClO4)4
2 AgCl + 2 ClOClO3 → 2 AgClO4 + Cl2
Applications
Chlorine perchlorate has been discovered in soil, surface water, and groundwater near sites where perchlorate salts were made, stored, or used. The salts are highly water-soluble, fully ionize in water, and produce the same perchlorate ion whether they are ammonium, sodium, or potassium salts.
The principal route of perchlorate exposure is ingestion of water from polluted public drinking water systems throughout the United States. Workers’ occupational exposure to ammonium perchlorate during commercial manufacturing or usage is greater than possible exposure from drinking water sources.
Perchlorate exposure occurs predominantly through inhalation of ammonium perchlorate dust, with systemic absorption through mucosal membranes in the respiratory and gastrointestinal systems. Some oral consumption is feasible, as is dermal contact, though considerable absorption of perchlorate through undamaged skin is uncommon.