For a few seconds, you will blank out or stare into space as a result of an absence seizure. Petit mal seizures are another name for certain types of seizures. Absence seizures are most prevalent in children and rarely produce long-term complications. These seizures are frequently precipitated by a period of hyperventilation.
Children between the ages of 4 and 14 are more likely to experience absence seizures. In a single day, a youngster may experience 10, 50, or even 100 absence seizures that go unreported. The majority of children with typical absence seizures are generally healthy. Absence seizures, on the other hand, can interfere with learning and impair concentration at school. This is why it is critical to receive quick treatment.
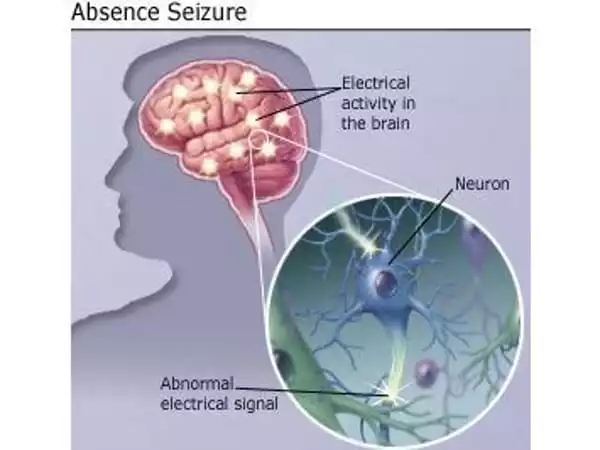
Absence seizures are characterized by a brief loss of consciousness lasting up to ten seconds, and they are followed with spike-wave discharges (SWDs) in cerebral electroencephalogram (EEG) recordings. The oscillatory activity that underpins cortical SWDs has been found to frequently come from a distinct concentration in several brain regions, such as the cerebral cortex, thalamus, or hippocampus. Researchers have gained new insights into the cellular and molecular causes of absence seizures, as well as prospective treatment alternatives.
Certain cerebellar areas could be stimulated to assist treat absence seizures. However, what happens at the cellular and molecular level in the brain in this type of epilepsy and how stimulation works are not well understood. Experiments with mice yielded fresh insights for Ruhr-Universität Bochum (RUB) researchers.
Dr. Jan Claudius Schwitalla
Certain cerebellar areas could be stimulated to assist treat absence seizures. However, what happens at the cellular and molecular level in the brain in this type of epilepsy and how stimulation works are not well understood. Experiments with mice yielded fresh insights for Ruhr-Universität Bochum (RUB) researchers. The findings were published in the journal “Cellular and Molecular Life Sciences” by a team lead by Dr. Jan Claudius Schwitalla and Professor Melanie Mark from the RUB Behavioral Neuroscience research department. They collaborated with colleagues from the Erasmus Medical Centers in Rotterdam and Utrecht, as well as Bonn, Münster, and München.
Abrupt loss of consciousness
Absence seizures (ASs) are characterized by pathological electrographic oscillations in the cerebral cortex and thalamus, which are called spike-and-wave discharges (SWDs). Subcortical structures, such as the cerebellum, may well contribute to the emergence of ASs, but the cellular and molecular underpinnings remain poorly understood.
Absence seizures, commonly known as petit mal seizures, affect around 1.5 million people worldwide. Patients experience a rapid loss of consciousness followed by a brief period of paralysis of behavior. Absence seizures are common in children aged four to twelve and are frequently misdiagnosed as daydreaming. They are associated with abnormal brain activity, which can be seen in brain activity recordings as spike-and-wave discharges (SWDs). The rhythmic and synchronized activation of nerve cells in the cerebral cortex and thalamus causes the distinctive activity pattern.
Because the nuclei located deep in the cerebellum have significant connectivity to multiple parts of the brain, researchers hypothesized that activating the cerebellar nuclei could be used to treat seizures. Other study groups’ experiments with animals revealed that such stimulation can really halt absence seizures. However, at the cellular and molecular levels, it is unknown what causes this effect.
Cerebellar stimulation against abnormal brain activity
The Bochum-based researchers studied with mice that suffer from absence seizures due to a deficiency of the P/Q-type calcium channel in cerebellar nerve cells. They discovered that cells in the cerebellar nuclei were firing improperly, and that stimulating these cells helped prevent subsequent SWDs. As a result, they stimulated the cerebellar nuclei using pharmacological or chemogenetic stimulation. For chemogenetic stimulation, a genetically engineered receptor is introduced into cells so that it can be activated by a specially designed chemical that is not ordinarily present in the brain. This enabled the researchers to gradually enhance the activity of the cerebellar nuclei cells, preventing subsequent SWDs in mice.
Furthermore, the researchers employed optogenetic stimulation to temporarily boost the activity of cells in the cerebellar nuclei and to interrupt ongoing SWDs. This method employs proteins from algae that can be activated by light to boost the activity of nerve cells. Overall, the study indicated that targeted stimulation of the cerebellar nuclei could become a therapeutic option for persons suffering from absence seizures.
The cerebellum has a dense network of outputs that originate in the CN and project to numerous thalamic nuclei as well as cortical and subcortical areas. As a result, electrical cerebellar cortex or CN stimulation was used to produce or stop several types of epilepsy in early animal investigations. Based on these experimental approaches, various human investigations attempted to harness the power of electrical cerebellar stimulation against epilepsy, with varying degrees of success.