Researchers at Simon Fraser University have discovered new ways to understand and control chemical interactions. Physical Review Letters published the results of their interdisciplinary approach.
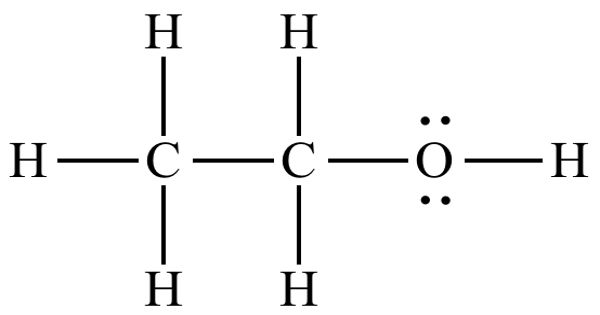
A chemical reaction occurs when the bonds between reactant molecules are broken and new bonds are established between product molecules, resulting in the formation of new material. Many activities involving chemical reactions that have been recognized and practiced for thousands of years include burning fuels, smelting iron, creating glass and pottery, brewing beer, and making wine and cheese.
Chemical processes, despite their complexity, frequently follow a set of basic steps as they advance. Miranda Louwerse, a Ph.D. student in chemistry at SFU, and David Sivak, a physics professor at SFU, discovered that the information offered by a reaction coordinate regarding how a reaction is developing exactly corresponds to how dissipating that coordinate is.
Their findings reveal a close relationship between two hitherto unrelated branches of physics: stochastic thermodynamics, which analyzes energy and information changes, and transition-path theory, which describes reaction mechanisms.
By establishing a relationship between these two areas, the researchers were able to develop a framework for quantifying the information about a reaction included in system dynamics, allowing them to gain a physical understanding of what it means for specific dynamics to be relevant for that reaction.
We weren’t looking for this. We found it in the course of studying something else. But it fits well in our broad research area understanding the interplay of energy, information, and dynamics in biological function at the molecular level.
David Sivak
This knowledge is especially important in assisting researchers in navigating large datasets. Chemical reactions are important in a variety of industries, customs, and even our daily lives. They are always occurring in our environment, such as rusting of iron, pottery, and wine fermentation, to name a few.
The researchers point out that developments in computing have made simulating complicated systems and chemical reactions easier than ever before, but these simulations can also generate a lot of useless data. This approach can aid researchers in distinguishing signal from noise, allowing them to follow the progression of a reaction in real-time.
Researchers and engineers will be able to better detect bottlenecks in the manufacture of chemicals as a result of this, making it easier to create interventions that will give them more control over reactions.
They will be able to produce chemicals faster, cheaper, and with less waste thanks to the guided design. It can also help researchers gain a better knowledge of how pharmaceutical medications act in the body, pointing them in the direction of producing drugs with less detrimental side effects.
This realization also opens up some exciting opportunities for more cross-disciplinary collaboration. Theorists can apply existing theory from one field to another by establishing a fundamental equivalence between basic notions in different fields.
This offers up the possibility of adapting methods for monitoring energy dissipation in order to find reaction mechanisms, which could lead to more information in the future.
“We weren’t looking for this,” Sivak says. “We found it in the course of studying something else. But it fits well in our broad research area understanding the interplay of energy, information, and dynamics in biological function at the molecular level.”
Physical changes must be differentiated from chemical processes. Changes in state, such as ice melting into water and water evaporating into vapour, are examples of physical changes. A substance’s physical attributes will change if it undergoes a physical change, but its chemical identity will not.
A chemical equation is a mathematical statement that represents the production of a product from reactants while also indicating the conditions under which the reaction was carried out. Reactants are on the left side of the equation, whereas products are on the right side, with one-headed or two-headed arrows connecting them.