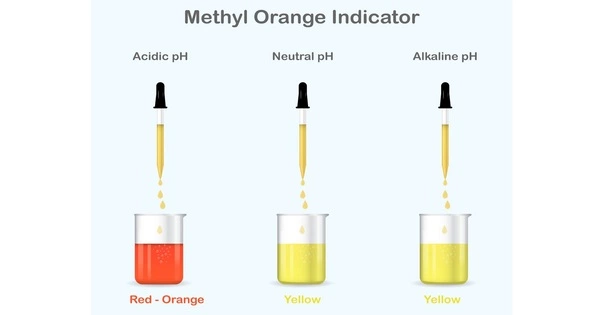
Methyl orange is a pH indicator that is frequently used in titration due to its clear and distinct color variation at different pH values. Methyl orange is red in the acidic medium and yellow in the basic medium. Because it changes color at the pKa of a mid-strength acid, it is commonly used in the acid titration. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it does have a sharp end point. When a solution becomes less acidic, methyl orange changes from red to orange, then to yellow—the opposite process occurs when the solution becomes more acidic.
Methyl Orange is a weak acid that degrades into orange neutral molecules when it comes into contact with water. The equilibrium is to the left in acidic conditions, and the concentration of neutral molecules is too poor to see the orange color.
Properties
Methyl orange has the property to color alkaline and neutral water yellow. The water turns red as soon as it becomes acidic. At pH 4.3, the transition occurs. The titration is performed with hydrochloric acid at a concentration of 0.1 mol/L if the solution is yellow.
- Chemical formula: C14H14N3NaO3S
- Molar mass: 327.33 g·mol−1
- Appearance: Orange or yellow solid
- Density: 1.28 g/cm3
- Melting point: > 300 °C (572 °F; 573 K) (not precisely defined)
- Boiling point: Decomposes
- Solubility in water: 5 g/L (20 °C)
- Solubility in diethyl ether: Insoluble
The color of alkaline solution is yellow, but when a mineral acid is added, the color changes to red. This color change is not caused by carbonic or other weak acids. As a result, this indicator can be used to titrate more powerful mineral acids in the presence of carbonic acid and weaker organic acids. The presence of a large amount of water causes the red color of a faintly acidic methyl orange solution to turn yellow, most likely due to hydrolytic dissociation.
Indicator colors
In a solution that decreases in acidity, methyl orange moves from the color red to orange and finally to yellow with the opposite occurring for a solution increasing in acidity.
Other indicators
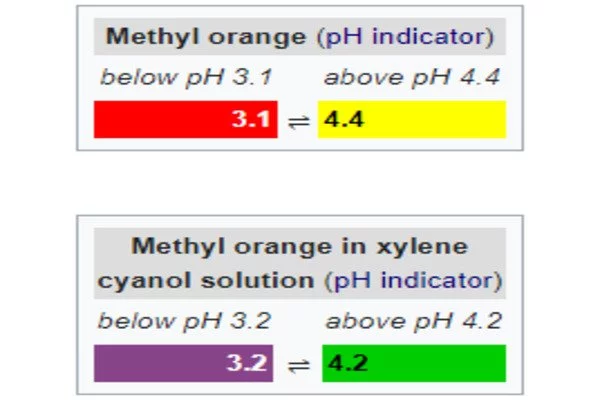
Modified (or screened) methyl orange, an indicator consisting of a solution of methyl orange and xylene cyanol, changes from grey-violet to green as the solution becomes more basic.
Preparation of Methyl Orange from Sulfanilic Acid
To produce methyl orange from sulfanilic acid and N, N-dimethylaniline, a diazonium coupling reaction was used, which is a common reaction for treating an aliphatic amine to yield a carbocation. When a primary aliphatic amine reacts with nitrous acid, an unstable diazonium salt is formed, which then loses N2 to form a carbocation.
The carbocation may then lose a proton to form an alkene, react with a nucleophile, or rearrange itself. In this case, the nucleophile is dimethylaniline. In the ortho position, the bulky dimethylamine substituent acts as a steric hindrance, causing attack in the para position. Because you’re making an azo dye, a Spectrophotometer can tell you how pure your product is.
Safety
Methyl orange has mutagenic properties.