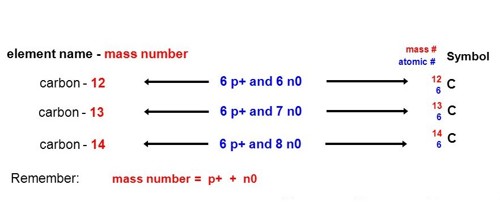
The atoms of a chemical element can exist in different types. These are called isotopes. They have the same number of protons (and electrons), but different numbers of neutrons. For example, an atom with 6 protons must be carbon, and an atom with 92 protons must be uranium. Different isotopes of the same element have different masses. The isotope of an element is defined by the nucleon number, which is the sum of the number of protons and the number of neutrons in the atomic nucleus.
Every chemical element has more than one isotope. Different isotopes of the same element have the same atomic number. They have the same number of protons. The atomic number is decided by the number of protons. Isotopes have different mass numbers, though, because they have different numbers of neutrons.
The word isotope, meaning at the same place, comes from the fact that isotopes are at the same place on the periodic table.
In a neutral atom, the number of electrons equals the number of protons. Isotopes of the same element also have the same number of electrons and the electronic structure. Atoms of elements with different numbers of neutrons are called “isotopes” of that element.
Stability
Atomic nuclei are protons and neutrons held together by the nuclear force.
Because protons are positively charged, they repel each other. Neutrons, which are neutral, stabilize the nucleus. Because they are in the nucleus, the protons are pushed slightly apart. This reduces the electrostatic repulsion between the protons. They still exert the attractive nuclear force on each other and on protons. One or more neutrons are necessary for two or more protons to bind into a nucleus. As the number of protons increases, so does the number of neutrons needed to have a stable nucleus.
There are 275 isotopes of the 81 stable elements. There are over 800 radioactive isotopes, some of which are natural and some synthetic. Every element on the periodic table has multiple isotope forms. In nature, some elements only have a single isotope. For example, fluorine-19 (19F) is the only stable isotope, of several, of fluorine. Other elements have many isotopes. For example, xenon has 9 isotopes. Of the 81 elements with a stable isotope, the largest number of stable isotopes for any element is ten (for the element tin).
Some isotopes are radioactive. These are called radioactive isotopes. Others are not radioactive. These are called stable isotopes. Stable isotopes either never decay or else decay very slowly. Radioactive isotopes undergo decay.
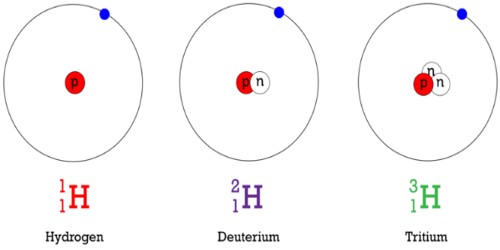
Hydrogen has three common isotopes. The most common isotope of hydrogen is called protium (1H). A hydrogen atom with an extra neutron (atomic mass of 2) is called deuterium (2H). Hydrogen with one proton and two neutrons (atomic mass of 3) is called tritium (3H). Protium and deuterium are stable isotopes, while tritium is a radioactive isotope.