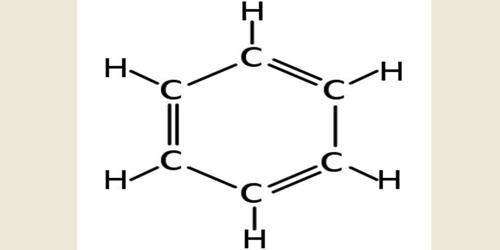
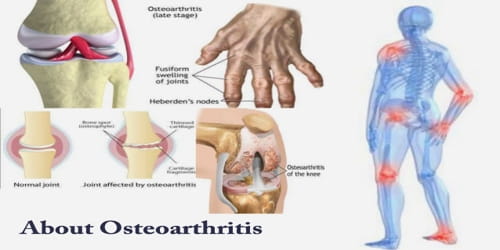
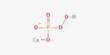
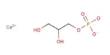
Cyclohexane is a cycloalkane with the molecular formula C6H12. It appears as a clear colorless liquid with a petroleum-like odor. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are precursors to nylon. It is an alicyclic hydrocarbon comprising a ring of six carbon atoms; the cyclic form of hexane, used as a raw material in the manufacture of nylon. Used to make nylon, as a solvent, paint remover, and to make other chemicals. It has a specific gravity of 0.78 and a flashpoint of -20° C and is highly flammable.
Cyclohexyl is the alkyl substituent of cyclohexane and is abbreviated Cy. Flashpoint -4°F. Density 6.5 lb/gal (less than water) and insoluble in water. It exhibits very poor solubility in water. The general rule for solubility in water is the factor of polarity. The solubility of water in cyclohexane, 0.01 % at 20°C. Regulatory and Safety Data. It is a symmetrical, hexagonal molecule that exhibits neither dipole-dipole interactions nor hydrogen bonding and therefore, it will not be soluble in water. It is saturated hydrocarbon (nonpolar compound) and water is a polar solvent, therefore, cyclohexanone uses as organic solvent for a nonpolar compound. Vapors heavier than air. It has a role as a non-polar solvent. Industrial cyclohexane can be produced by two methods. The first is the catalytic hydrogenation of benzene using rhodium on carbon, and the second method is via fractional distillation of petroleum.
Cyclohexane is relatively non-toxic and does not have adverse effects on the blood like those of benzene. It occurs naturally in petroleum crude oil, volcanic gases, and cigarette smoke. It causes depression of the central nervous system and, at high concentrations, has narcotic effects. For transportation purposes, it is classified as hazard class 3 and packing group II and it should be labeled as an irritant, and harmful if swallowed or inhaled. It has a mild odor and is insoluble in water but soluble in alcohol, ether, acetone, benzene, and ligroin. The purity level required for the use of cyclohexane, especially for its oxidation, is higher than 99%. This purity can be obtained by the benzene hydrogenation technique. It is used predominately in the nylon industry where approximately 90% of it is consumed in the industrial production of adipic acid and caprolactam, which are themselves used to generate nylon6 and nylon6.6.