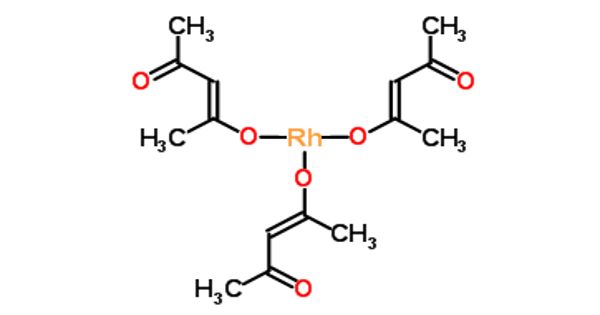
Rhodium acetylacetonate is the coordination complex with the formula Rh(O2C5H7)3, which is sometimes known as Rh(acac)3. It is a Rhodium source that is soluble in organic solvents. The molecule has D3-symmetry. Rhodium acetylacetonate, as the organic solvent-soluble source of rhodium metal ions, can be used to prepare monodisperse size/shape-controlled Rh nanocrystals.
Rhodium acetylacetonate is useful for hydrosilylation of a variety of organic substrates like alkenes, terminal or internal acetylenes, conjugated dienes, alpha, beta-unsaturated aldehydes, and allylic compounds.
Properties
It is a yellow-orange solid that is soluble in organic solvents. Ultraviolet-visible absorption spectra show absorptions at 320 nm, 260 nm, and 210 nm. Density measurements yielded a value of 1.75 g/cm3. Thermal characteristics were evaluated using differential thermal analysis (DTA). It was found that the compound is not volatile, decomposing upon heating.
- Compound Formula: Rh(O2C5H7)3
- Molecular Weight: 400.23
- Appearance: Yellow to Dark Green Powder
- Melting Point: 263-264 °C
- Boiling Point N/A
- Exact Mass: 400.039317
- Monoisotopic Mass: 400.039317
If heating rates are rapid enough,e.g. > 2° C/min, melting at 267° C can be observed. If heating rates are slower, decomposition is complete below the melting point.

It is prepared from RhCl3(H2O)3 and acetylacetone. The high purity acetylacetonate anion complexes by bonding each oxygen atom to the metallic cation to form a chelate ring; because of this property, acetylacetonates are commonly used in various catalysts and catalytic reagents for organic synthesis, including the fabrication of various shapes of carbon nanostructures. The complex has been resolved into individual enantiomers by separation of its adduct with dibenzoyl tartaric acid.
Uses
It is used in catalytic reagents for organic synthesis. It is useful for hydrosilylation of a variety of organic substrates like alkenes, terminal or internal acetylenes, conjugated dienes, alpha, beta-unsaturated aldehydes, and allylic compounds. Rh nanocrystal monolayers have been reported to show high activity for hydrogenation of vinyl cyclohexene to selectively produce ethylcyclohexene. The size/shape-controlled nanocrystals find a variety of applications in oil refining processes, chemical synthesis, and fuel cell technology.