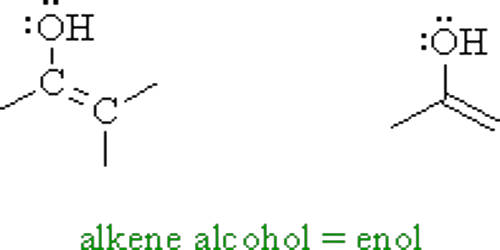Enol is an organic compound that contains a hydroxyl group bonded to a carbon atom having a double bond and that is usually characterized by the grouping C=C(OH). Enols, or more formally, alkenols, are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. In general, enols are unstable compounds and they are in equilibrium with a more favorable carbonyl group. The terms enol and alkenol are portmanteaus deriving from “-ene”/”alkene” and the “-ol” suffix indicating the hydroxyl group of alcohols, dropping the terminal “-e” of the first term. Enols are isomers of aldehydes or ketones in which one alpha hydrogen has been removed and replaced on the oxygen atom of the carbonyl group. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, H+. The resulting molecule has both a C=C (-ene) and an –OH (-ol) group, so it is referred to as an enol. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate. Enolates are the conjugate bases or anions of enols (like alkoxides are the anions of alcohols) and can be prepared using a base. The corresponding enolate ion is formed by dissociating a hydrogen atom from the α carbon atom and then rearranging the electron “locations” slightly.
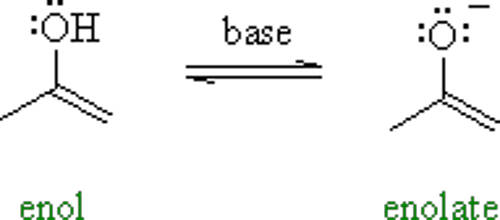
The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, the generation of the enol often is accompanied by “trapping” or masking of the hydroxy group as an ether, such as a silyl enol ether. Enol formation is called “enolization”. Enol acetates and alkyl enol ethers can be α-hydroxylated through peracid epoxidation in a process analogous to that for silyl enol ethers. One possible reason is that the acyl group of an enol ester has a weaker coordinating ability to the metal catalyst than that of the corresponding enamide substrate.