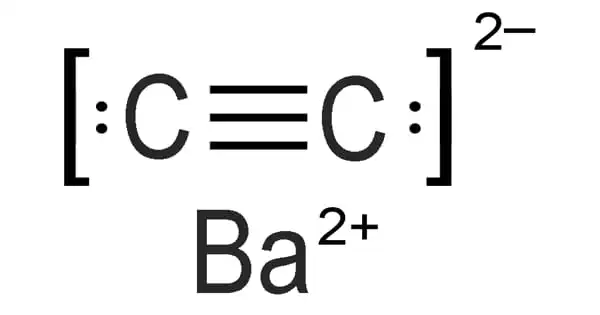Barium carbide, also known as BaC2, is a chemical compound in the carbide family having the formula BaC2. Boron carbide powder is primarily made by combining carbon with B2O3 in an electric arc furnace, carbothermal reduction, or gas phase processes. B4C powders for commercial usage are typically processed and filtered to remove metallic contaminants.
Boron carbide powder is utilized as an abrasive in polishing and lapping applications, as well as a loose abrasive in cutting applications such as water jet cutting, due to its high hardness. It is also useful for diamond tool dressing.
Properties
Barium carbonate is a white powder. It is insoluble in water and soluble in most acids, with the exception of sulfuric acid. It has a specific gravity of 4.275. It has a molecular weight of 161.348 g/mol. It is a gray cubic to rhombohedral crystal with a density of 3.74 g/cc. It is toxic by ingestion.
- Molecular Weight: 161.35
- Appearance: black crystalline solid
- Melting Point: N/A
- Boiling Point: N/A
- Density: 3.75
- Specific gravity: 4.275
- Solubility in H2O: N/A
- Form: gray tetragonal crystals
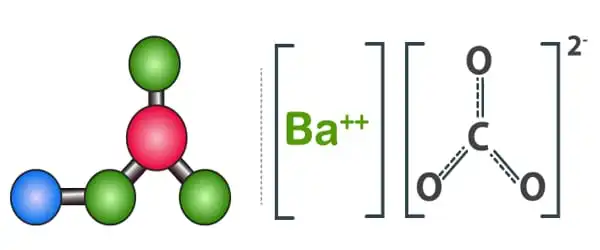
Preparation
By reducing barium carbonate powder with metallic magnesium in the presence of carbon-14, barium carbide can be produced as an impure chemical. Carbon-14 barium carbide can also be produced by reducing 14C carbon dioxide with heated barium metal at 600°C. These procedures are used because of their high yield and the fact that the carbide is used to produce acetylene. Carbon-14 is not something that should be discarded. It can also be made by heating a mixture of barium amalgam and carbon powder in a hydrogen current. The pure chemical is made by reducing Barium oxide with Carbon at high temperatures.
Reaction
Barium carbide reacts in the same way as calcium carbide, although it is more fusible. When exposed to high temperatures, barium evaporates, leaving behind graphite crystals. It can also absorb carbon in a high-temperature solution.
Barium carbonate powder is dense and white, and it is made from either barite (BaSO4) or barium chloride. Following that, a precipitation procedure is employed to obtain the carbonate form. There are various crystalline forms of BaCO3, the most stable of which is alpha.
Thermally, barium carbonate is very stable and does not quickly dissociate unless at least some CO is present in the kiln atmosphere (i.e. reduction).
Uses
Barium Carbide is used as a carbonate source to produce acetylene, ammonia. It is also used in paints and colorants and in the electronic coating. Other applications include ceramic tooling dies, precision toll parts, evaporating boats for materials testing, and mortars and pestles.
Hazards
Barium carbide can cause damage to the GI tract and irritation in the skin and eyes.