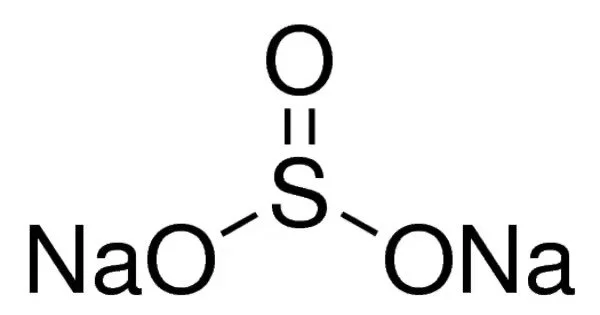
Sodium sulfite (sodium sulphite) is an inorganic compound with the chemical formula Na2SO3. It is a white, odorless solid that is soluble in water. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air. It is commonly used as a reducing agent, preservative, and antioxidant in the food industry. It can also be used in the photographic industry, as a water softener, and in the production of paper.
Properties
- Molar mass: 126.043 g/mol
- Appearance: White solid
- Odor: Odorless
- Density: 2.633 g/cm3 (anhydrous); 1.561 g/cm3 (heptahydrate)
- Melting point: 33.4 °C (92.1 °F; 306.5 K) (dehydration of heptahydrate); 500 °C (anhydrous)
- Boiling point: Decomposes
- Solubility in water: 27.0 g/100 mL water (20 °C)
- Solubility: Soluble in glycerol
- Insoluble: in ammonia, chlorine

Preparation
Sodium sulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a white solid. With more SO2, the solid dissolves to give the disulfite, which crystallizes upon cooling.
SO2+2NaOH ⟶ Na2SO3+H2O
Sodium sulfite is made industrially by treating sulfur dioxide with a solution of sodium carbonate. The overall reaction is:
SO2+Na2CO3 ⟶ Na2SO3+CO2
Applications
The pulp and paper industry is the primary user of sodium sulfite. It has also been used in the thermomechanical conversion of wood to fibers (defibration) for the manufacture of medium-density fiberboards (MDF). It is used in the photographic industry to protect developer solutions from oxidation and (as hypo clear solution) to wash fixer (sodium thiosulfate) from film and photo-paper emulsions. It is also used as an oxygen scavenger agent in water fed to steam boilers to avoid corrosion problems.
In the food industry, it is used to prevent browning and spoilage in fruits and vegetables, to maintain the color of seafood, and to preserve the flavor of wine. It can also be used to prevent the growth of bacteria in some processed foods. In the photographic industry, it is used as a fixing agent to remove unexposed silver halide from photographic prints and negatives. It can also be used as a preservative in developing solutions.
Sodium sulfite is also used as a water softener in industrial and household applications, as it can remove calcium and magnesium ions from water. In the production of paper, sodium sulfite is used to bleach pulp and remove impurities.
Safety
While sodium sulfite is generally considered safe, it can cause respiratory and skin irritation in some people. It can also be toxic if ingested in large amounts. Therefore, it is important to handle sodium sulfite with care and follow proper safety precautions.