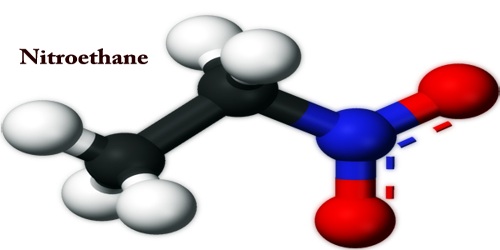
Nitroethane is a colorless oily liquid, with an unpleasant odor; it is an organic compound having the chemical formula C2H5NO2 and CAS number 79-24-3. Flashpoint of 82°F; Decomposes above 350°F; Density 1.052 g/cm3. The product of ethanol, acetone, and ethyl ether is totally miscible. This is just slightly water-soluble. Nitroethane is an oily liquid and is similar in many respects to nitromethane at normal temperature and pressure. Pure nitroethane is colorless and has a fruity flavor, with a solubility at 20 ° C of 4.6 g/100 ml.
Nitroethane is produced industrially by treating propane with nitric acid at 350–450 °C. The solubility in water is 4.5mL/100mL (20℃), but the water solubility in this product is 0.9mL/100mL (20 ℃). Steam and air can form explosive mixtures; the explosion limit is 3%~5% (in volume). Nitroethane is a nitroalkane substituted by nitro group ethane, and it is a tautomer of an aci-nitroethane. This exothermal reaction produces four nitroalkanes of industrial significance: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane.
Because of the chemical properties of nitroethane, nitrocellulose and cellulose acetate, vinyl and alkyd resin, wax, graase, fat and dye solvent can be used as propellant and fuel additives as well as intermediate pharmaceutical and organic synthesis agents. The reaction involves free radicals, such as CH3CH2CH2O, which arise via homolysis of the corresponding nitrite ester. Nitroethane can be prepared by direct gas phase nitration of ethane or produced by the effect of α-acid chloride and sodium nitrite.
Nitromethane is that the simplest organic nitro compounds, may be a colorless oily with a faint aromatic smell of transparent liquid at temperature, with a bigger polar, flammable, poisonous, and explosive. Alternatively, nitroethane may be produced by the Victor Meyer reaction of haloethanes like chloroethane, bromoethane, or iodoethane with silver nitrite in ether or THF. The Kornblum modification of this reaction uses nitrite in either a dimethyl sulfoxide or dimethylformamide solvent.
Nitroethane condenses with two equivalents of formaldehyde to offer, after hydrogenation, 2-amino-2-methyl-1, 3-propanediol, which successively condenses with monounsaturated fatty acid to administer oxazoline, which protonates to present a cationic surfactant. As negative ions of carbon nucleophiles to the body, nitromethane is an occurrence conjugate addition with α,β-unsaturated carbonyl compounds under the catalysis of alkali, which is alleged Michael reaction.
The majority of applications of nitroethane include being an intermediate in organic synthesis reactions. It can be used as fuel. This has been used as a component in artificial nail removers and in overhead ceiling sealant sprays in cosmetics applications. Contact with the skin can lead to contact dermatitis; high-concentration inhalation has an anesthesia effect. Nitroethane was previously used successfully as a chemical feedstock (precursor ingredients) in laboratories for the synthesis of multitudes of gear and goods. If mistakenly swallowed, emetic, lavage, give the medical gaze, symptomatic treatment; severe cases, don’t induce vomiting immediately, should be sent to the hospital for treatment.
Kids were poisoned by the ingestion of a remover of artificial nails. In the event of extended or chronic exposure, the compound is believed to cause genetic damage and harm to the nervous system. Nitroethane is readily converted to acetaldehyde, nitrite, nitrate, hydrogen peroxide, and dinitroethane through glucose oxidase in vitro.
Information Sources: