Magnesium hydroxide, Mg(OH)2, is a crystalline white to off-white powder with a specific gravity of 2.4 and a hardness of Mohs of about 3.0. It is a magnesium hydroxide solution with anti-acid and laxative properties. It is a white powder formed by the addition of lime milk from seawater in large quantities (calcium hydroxide). In nature, it occurs as the mineral brucite. It is a white solid with low solubility in water (Ksp = 5.61×10−12). In the manufacture of magnesium alloy, it is the main raw material and has been used as a fire-retardant additive. It forms a suspension of water known as magnesia milk, which has long been used as an anti-acid and a laxative.
When heated above 450 °C, magnesium hydroxide loses 30.9 percent of its mass as water vapor. It is soluble in a solution of dilute acid and ammonium chloride, but nearly insoluble in water and alcohol. Combining a solution of several magnesium salts with alkaline water produces solid Mg(OH)2 precipitation:
Mg2+ + 2 OH− → Mg(OH)2
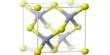
(Structure of Magnesium Hydroxide)
The action of hydrochloric acid on magnesium hydroxide produces magnesium chloride, MgCl2, a colorless, delicate (water-absorbing) material used in the manufacture of magnesium metal, in the manufacture of heavy-duty flooring cement, and as an additive in the manufacture of textiles. It is used for the manufacture of plastics and filled (co)polymer compounds based on elastomers, thermosets, thermoplastics like those based on polyvinylchloride, polyamides, polystyrene, polyethylene, polypropylene, polyethylene terephthalate, ultrathene, as a highly efficient non-toxic inorganic flame retardant, filler, and smoke suppressant additive, and so on, in paper and cardboard industry, as a mild neutralizing agent for waste and natural water treatment, as a raw material in the chemical and pharmaceutical industry.
In addition, magnesium hydroxide has properties that inhibit smoke and flame retardant and is thus commercially used as a fire retardant. It can also be used topically to relieve cancer sores or as a deodorant (aphthous ulcers). It is also used in the manufacture of tofu to coagulate soy milk. Much of the industrially produced Mg(OH)2 and the limited quantity removed is converted to fused magnesia (MgO). As it is both a poor electrical conductor and an outstanding thermal conductor, Magnesia is valuable.
Magnesium hydroxide is used as an absorbent for desulfurizing flue gas. Most flue gas desulfurization used sodium hydroxide technique, calcareous gypsum technique, in the 20th century, before the 1970s. As the Mg2+ ion is involved with the vital biological polyphosphate compounds DNA, RNA, and adenosine triphosphate, magnesium is essential for all living cells (ATP). In order to function, many enzymes rely on magnesium. Magnesium hydroxide is medically used to regulate gastric acid and laxatives, as a mineral additive, as a color preservation agent, as a desiccant, as an alkali agent, and as a processing aid for sugar.

(Example of Magnesium Hydroxide)
Via the inclusion of aluminum hydroxide, some magnesium hydroxide products sold for antacid use such as Maalox) are designed to reduce excessive laxative effects by inhibiting the contractions of smooth muscle cells in the gastrointestinal tract, counterbalancing the contractions caused by the osmotic effects of magnesium hydroxide. One of the components of the universal remedy for poisoning is magnesium hydroxide or milk of magnesia. Solid magnesium hydroxide has smoke suppressing and flame retardant properties, much like aluminum hydroxide. This property is attributable to the endothermic decomposition it undergoes at 332°C (630°F):
Mg(OH)2 → MgO + H2O
Magnesium is also an important component of chlorophyll, the green pigment present in nearly all plants, algae, and cyanobacteria. Additives to cable insulation, insulation plastics, roofing, and different flame retardant coatings are common uses of magnesium hydroxide as a flame retardant. Magnesium hydroxide is mainly excreted in the urine by the kidneys. As the kidneys play a significant role in their clearance, individuals with kidney failure are at risk of long-term hypermagnesemia, as sufficient quantities of magnesium may not be excreted. Magnesium hydroxide alone (magnesia milk) is often used in small animals as an oral laxative.
Information Sources: